
- |<
- <
- 1
- >
- >|
-
Yoshinori Nakano, Motoyasu Miura, Noriyuki Namiki, Shinya Uchida2024 年 72 巻 11 号 p. 936-943
発行日: 2024/11/01
公開日: 2024/11/01
ジャーナル オープンアクセス HTMLOrally disintegrating tablets (ODTs) can be easily swallowed without drinking water and are a convenient dosage form for the elderly and infirmed patients, as well as other patients, such as businesspeople. A major challenge in the development of ODTs is masking the unpleasant taste of the drug, which can make ODTs palatable. Flavors are often used for taste-masking. A few comprehensive studies have been conducted on the selection of suitable flavors for ODTs. This study aimed to evaluate the effects of different flavors on the taste sensation of ODTs using a visual analog scale (VAS). Sixteen flavors were studied for their effects on the taste sensation of pioglitazone ODTs in a randomized single-blind study involving the gustatory sensation testing of ODTs. Healthy volunteers were enrolled and asked to periodically evaluate the bitterness, sweetness, astringency, sourness, and overall palatability of ODTs, both during and after disintegration in the oral cavity, using the VAS. Most flavors improved the sweetness of ODTs without the addition of a sweetener, and some suppressed bitterness, astringency, and sourness. In particular, blueberry, and yoghurt flavors significantly improved sweetness and overall palatability during disintegration of the pioglitazone ODT. The approach of using VAS score analysis was effective in selecting the most suitable flavor for improving the overall palatability of ODT. Furthermore, the addition of a suitable flavor can successfully mask the unpleasant taste of the drug and effectively improve the overall palatability of ODT.
 抄録全体を表示PDF形式でダウンロード (971K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (971K) HTML形式で全画面表示 -
Hue Thi Buu Bui, Zin Paing Htoo, Quang Vinh Hong, Hieu Trong Le, De Qu ...2024 年 72 巻 11 号 p. 944-949
発行日: 2024/11/07
公開日: 2024/11/07
ジャーナル オープンアクセス HTML
電子付録Benzimidazoles have a broad spectrum of biological and pharmacological properties, including anticancer activity. This study reports the facile synthesis and cytotoxic evaluation of twenty-eight 1,2-disubstituted benzimidazoles (6a–β), based on condensation reactions between N-benzyl o-phenylenediamine and benzylamine. The reactions were solvent-free, with the use of Na2S2O5 as an inexpensive and environmentally friendly oxidizing agent, and progressed rapidly. Cytotoxicity assessments using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay were performed against the A549, HeLa, and MCF-7 cell lines for all synthesized compounds (6a–β). Among them, 6j, 6k, 6l, and 6n displayed good activities against the A549 and MCF-7 cell lines. These compounds possessed IC50 values ranging from 2.55 to 4.50 µM, corresponding to 1.4-fold to 2.4-fold stronger potencies than that of the positive control 5-fluorouracil (5-FU) (IC50 = 6.08 µM) against MCF-7 cells, while 6k (IC50 = 3.22 µM) was consistent with 5-FU on the A549 cell line (IC50 = 3.77 µM). Structure–activity relationship analyses revealed the 3-pyridinyl moiety at C-2 and the CH3, OCH3, or 1,3-dioxolyl groups on the benzene ring at the N-1 position of the benzimidazole heterocycle as key structural features effectuating the observed cytotoxicities.
 抄録全体を表示PDF形式でダウンロード (835K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (835K) HTML形式で全画面表示 -
Pathuri Raghuveer, Dadi Shanthi, Thummala Uday Kumar, Potti Lakshmana ...2024 年 72 巻 11 号 p. 950-960
発行日: 2024/11/14
公開日: 2024/11/14
ジャーナル オープンアクセス HTMLThe development of polymeric nanoparticles (NPs) from preformed polymers usually requires the use of organic solvents and is more expensive. Hence, in this work, the development of polymeric nanoparticles by in situ aqueous dispersion polymerization from the monomers was set as an objective. Acrylonitrile monomer based polymeric NPs comprising Lamivudine (LMV) as a model drug were prepared using the aqueous dispersion polymerization technique. A quality by design approach was applied to optimise various formulation and process factors viz. monomer concentration, initiator concentration, stabilizer concentration and polymerization temperature. Polymerization time (PT), entrapment efficiency (EE), particle size (PS), and drug release rate constant (k) were taken as the responses to define the quality of the prepared NPs. Design of experiments analysis followed by optimization was performed to identify the optimized combination of the factors. Later, the optimized formulation was studied for the physical state of the LMV in the nanoparticles, surface morphology of the NPs and cytotoxicity studies. The optimized formulation was found to have 91.7 min. of PT, 81.4% of EE, 253 nm of PS and a k value of 0.262 h−1 (18 h to release 99%). The cytotoxicity studies indicated that the NPs were highly safe to use. These results altogether inferred that LMV contained NPs were developed effectively from the acrylonitrile monomer by the aqueous dispersion polymerization method.
 抄録全体を表示PDF形式でダウンロード (7904K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (7904K) HTML形式で全画面表示 -
 Hidetomo Yokoo, Hinata Osawa, Kosuke Saito, Yosuke Demizu2024 年 72 巻 11 号 p. 961-965
Hidetomo Yokoo, Hinata Osawa, Kosuke Saito, Yosuke Demizu2024 年 72 巻 11 号 p. 961-965
発行日: 2024/11/14
公開日: 2024/11/14
ジャーナル オープンアクセス HTML
電子付録Proteolysis-targeting chimeras (PROTACs) have attracted attention as an innovative drug modality that induces the selective degradation of target proteins. This technology shows higher activity than conventional inhibitors and holds great potential in the field of drug discovery. Optimization of the linker is essential for PROTACs to achieve sufficient activity, particularly with regard to cell membrane permeability. However, the correlation between membrane permeability and the activity of PROTACs has not been fully explored. To address this, we established a new molecular design approach to remove the linker and optimize PROTAC structure. These PROTAC compound groups were used to analyze the correlation between membrane permeability and activity using LC-tandem mass spectrometry (LC-MS/MS). Results revealed that the degradation activity of PROTACs fluctuates with increasing membrane permeability and changes in response to linker optimization, while sufficient proteolytic activity can be retained. These findings demonstrate the importance of considering the balance between membrane permeability and activity in PROTAC design and provide a new strategy for developing more effective PROTACs.
 抄録全体を表示Editor's pick
抄録全体を表示Editor's pickThis study examines the relationship between membrane permeability and the intracellular degradation activity of Proteolysis-Targeting Chimeras (PROTACs). Using hematopoietic prostaglandin D synthase (H-PGDS) as a model protein, this study investigated how linker length impacts the performance of PROTACs. The findings reveal that membrane permeability and H-PGDS degradation activity are influenced by linker properties, with shorter linkers shown to enhance both permeability and activity under specific conditions. This study highlights the importance of linker optimization in PROTAC design and provides strategies to balance molecular weight, permeability, and efficacy. These insights contribute to the advancement of PROTACs as effective therapeutic agents.
PDF形式でダウンロード (948K) HTML形式で全画面表示 -
 Yousuke Yamaoka, Ryo Nishina, Ken-ichi Fujita, Kiyosei Takasu2024 年 72 巻 11 号 p. 966-969
Yousuke Yamaoka, Ryo Nishina, Ken-ichi Fujita, Kiyosei Takasu2024 年 72 巻 11 号 p. 966-969
発行日: 2024/11/15
公開日: 2024/11/15
ジャーナル オープンアクセス HTML
電子付録This study explores the synthesis of unique furanocembranoid-type marine diterpenoid, providencin. Providencin features a highly oxidized structure with two furan rings, two oxirane rings, and a bicyclo[12.2.0]hexadecane framework. Its potential as a lead compound for drug development has drawn attention to its total synthesis, particularly focusing on the challenging right-half segment involving a highly substituted cyclobutane ring. We developed a novel synthetic strategy for the fragment using a [2 + 2] cycloaddition reaction of lithium ynolates with α,β-unsaturated lactone, successfully constructing a bicyclic cyclobutene structure. Stereoselective hydrogenation of cyclobutenes was achieved by using Crabtree’s catalyst under high pressure H2 atmosphere. After further transformation, the synthesis of the furan-substituted cyclobutanol fragment having a formyl side chain was accomplished.
 抄録全体を表示Editor's pick
抄録全体を表示Editor's pickProvidencin, a marine diterpenoid with a unique structure featuring two furan and oxirane rings along with a bicyclo[12.2.0]hexadecane skeleton, has attracted interest as a potential lead compound for drug development. The authors successfully synthesized its challenging right-half segment, a furan-substituted cyclobutane, in 10 steps. Notable advances include the [2+2] cycloaddition of lithium ynolates to construct a poly-substituted cyclobutene, followed by stereoselective hydrogenation using Crabtree’s catalyst. This streamlined approach represents a major milestone in the synthesis of multi-functional cyclobutanes and represents a significant step forward in total synthesis research.
PDF形式でダウンロード (764K) HTML形式で全画面表示 -
Zeping Luo, Liwei Pan2024 年 72 巻 11 号 p. 970-978
発行日: 2024/11/15
公開日: 2024/11/15
ジャーナル オープンアクセス HTML
電子付録This study aims to design and synthesize a series of novel formononetin (FMN) derivatives and explore their protective effects on oxygen glucose deprivation/relapse (OGD/R) damage to H9C2 cells, along with their molecular regulatory mechanisms. The OGD/R model was established to simulate myocardial ischemia–reperfusion injury. The protective effects of these novel compounds on H9C2 cells were evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method, while the apoptosis rate, myocardial enzyme activity, and autophagy reaction post-compound treatment were assessed using kit-based methods. The formation of autophagosomes in H9C2 cells was observed via transmission electron microscopy, and the expression levels of autophagy-related proteins phosphatidylinositol 3-kinase (PI3K), Akt, Beclin-1, and P62 were determined using Western blotting. The experimental findings demonstrated that compounds 1–6 (C1–6) exhibited varying degrees of protective effects on damaged H9C2 cells, generally outperforming the parent compound FMN. Among these compounds, C4 demonstrated the most significant activity, even surpassing the positive control drug diltiazem. Further mechanistic investigations revealed that C4 could mitigate apoptosis rates, reduce the activity of myocardial enzyme (such as aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and CK), diminish the number of autophagic vesicles, and restore excessive autophagy. Additionally, C4 exerted its protective effects by downregulating the expression of autophagic proteins PI3K, Akt, Beclin-1, P62, LC3 and ATG12. These results indicated that C4 regulates autophagy through the PI3K/Akt/Beclin-1 signaling pathway, thereby exerting a protective effect on cardiomyocytes. Therefore, C4 emerges as a potential myocardial protective drug, offering a new research direction and strategy for the treatment of myocardial ischemia–reperfusion injury.
 抄録全体を表示PDF形式でダウンロード (6004K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (6004K) HTML形式で全画面表示 -
Yonglian Li, Zonglin You, Vincent Kam Wai Wong, Min Chen, Kun Zhang, W ...2024 年 72 巻 11 号 p. 979-988
発行日: 2024/11/21
公開日: 2024/11/21
ジャーナル オープンアクセス HTMLNaproxen, widely used to treat anti-inflammatory diseases, would cause serious of side effects. Based on the biological activities of cinnamic acid, naproxen derivatives containing cinnamic acid were designed, synthesized and used to enhance their anti-inflammatory activities and safeties. The results investigated that thirty novel naproxen derivatives had inhibitory effects on the nitric oxide (NO) release in RAW264.7 macrophage cells. A majority of naproxen derivatives showed the lower degree of cytotoxicity than that of naproxen. In vitro studies revealed that A22 (IC50 = 7.38 ± 1.96 µM) blocked the activation of nuclear transcription factor κB (NF-κB) signaling pathway and pyrin domain containing protein 3 (NLRP-3) inflammasome in a concentration dependent manner, thereby down-regulating the expression of pro-inflammatory cytokines, such as interleukin (IL)-1β, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Docking studies confirmed that A22 exhibited a well-fitting into the NLRP3 active site. Accordingly, A22 might be a novel NLRP3 inhibitor to treat inflammatory diseases.
 抄録全体を表示PDF形式でダウンロード (4770K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (4770K) HTML形式で全画面表示 -
Masaki Higashino, Kiyohiko Sugano2024 年 72 巻 11 号 p. 989-995
発行日: 2024/11/21
公開日: 2024/11/21
ジャーナル オープンアクセス HTMLThe purpose of the present study was to compare phosphate buffer (PPB) and bicarbonate buffer (BCB) solutions as dissolution test media for predicting the bioequivalence (BE) of an immediate-release (IR) formulation. Febuxostat was used as a model of free acid drugs. One reference formulation (RF) and three test formulations (TF) were employed in this study. The clinical BE studies involved 18 to 24 healthy adult volunteers. Each formulation was orally administered in the fasted state. The compendial paddle apparatus was used for the dissolution tests (500 mL, 37 °C, 25 or 50 rpm). BCB (10 mM, pH 6.8, 140 mM NaCl) and PPB (2.5 to 25 mM, pH 6.8, 140 mM NaCl) were used as dissolution media. The pH value of BCB was maintained by the floating lid method. In the clinical BE studies, two TFs were BE to RF, whereas one TF was non-BE. At a paddle speed of 50 rpm, RF and TFs showed little or no difference in the dissolution profiles in all buffer solutions. At 25 rpm, the dissolution profiles in 2.5 mM PPB and 10 mM BCB were consistent with the clinical BE results. The in vitro–in vivo correlation between Cmax ratio and dissolved% ratio at each time point was highest for 10 mM BCB at 25 rpm. These results suggest that the use of BCB increases the BE predictability of dissolution tests.
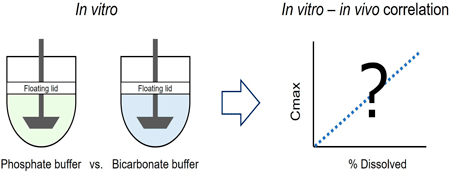 抄録全体を表示PDF形式でダウンロード (2796K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (2796K) HTML形式で全画面表示 -
Nguyen Duc Tuan, Bui Thi Hong Phuong, Tran Thanh Dao, Vo Thi Kim Khuye ...2024 年 72 巻 11 号 p. 996-1004
発行日: 2024/11/26
公開日: 2024/11/26
ジャーナル オープンアクセス HTML
電子付録WHO declared the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus as a global pandemic (coronavirus disease 2019 (COVID-19)) in March 2020. Molnupiravir (MPV) is an oral antiviral drug that received use authorization for mild to moderate COVID-19 treatment in adults. However, the global and national drug testing and research of MPV is still difficult due to lack of standards and validated procedures. Therefore, this work aims to synthesize the standard substance of two main molnupiravir impurities, N-hydroxycytidine (NHC) and dimethyl dioxol (DMDO), followed by development of a HPLC-photo diode array (PDA) for their simultaneous analysis. The procedure was validated in compliance with the international pharmaceutical analysis guideline (ICH), and employed to test these compounds in molnupiravir on the market. As a result, NHC and DMDO were successfully synthesized by a hydrolysis in an alkaline environment, and acetalization in an acid environment with very high yields of 83.76 and 73.51%, respectively, along with the purities of over 99%. NHC was detected below the allowable threshold whereas DMDO could not be detected in our samples. The findings show the high applicability of our synthesis and determination procedures in the large-scale production and quality control of impurities of commercial molnupiravir medicines.
 抄録全体を表示PDF形式でダウンロード (1713K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (1713K) HTML形式で全画面表示 -
 Toru Ekimoto, Masami Nomura, Yuri Saito, Minami Suzuki, Tsutomu Yamane ...2024 年 72 巻 11 号 p. 1005-1013
Toru Ekimoto, Masami Nomura, Yuri Saito, Minami Suzuki, Tsutomu Yamane ...2024 年 72 巻 11 号 p. 1005-1013
発行日: 2024/11/26
公開日: 2024/11/26
ジャーナル オープンアクセス HTMLToll-like receptors (TLRs) play central roles in innate immune defense against infection by binding to microbial molecules. TLR7 and TLR8 are highly homologous sensors with an RNA ligand preference for single-stranded RNA (ssRNA). Recent works reveal that these TLR sense degradation products of RNA at two distinct binding sites, designated 1st site and 2nd site, rather than long ssRNA. The highly conserved 1st site is responsible for the binding of nucleosides and the 2nd site confers the oligonucleotide binding. Binding of the oligonucleotide at the 2nd site synergistically enhances the affinity for nucleoside to the 1st site. However, it remains unclear why these ligands synergistically activate TLR7 and TLR8. Here, we performed a molecular dynamics (MD) calculation and successive decomposition analysis to clarify what this synergistic effect is derived from. We demonstrated that the main factor of the synergistic effect during the TLR7 and TLR8 activation processes was the lowering of the LRR dimerization barrier, mainly achieved by the reduction of the electrostatic repulsion with the oligonucleotide binding at the 2nd site.
 抄録全体を表示Editor's pick
抄録全体を表示Editor's pickToll-like receptor 7 and 8 are single-stranded RNA (ssRNA) sensors in the innate immune system. They recognize the degradation products of ssRNAs, nucleoside and short oligonucleotides, at two distinct ligand-binding sites. The binding to the 2nd site of the oligonucleotide allosterically increases the affinity of the 1st site toward the nucleoside, but its mechanism remains largely unknown. In this manuscript, the authors clarified this synergistic effect from the computational science approach. The oligonucleotide binding at the 2nd site reduces the electrostatic repulsion, resulting in the lowering of the LRR dimerization barrier, thus increasing the affinity of the 1st site for the nucleoside.
PDF形式でダウンロード (8809K) HTML形式で全画面表示 -
Léa Rubira, Jade Torchio, Juliette Fouillet, Johanne Vanney, Cyril Fer ...2024 年 72 巻 11 号 p. 1014-1023
発行日: 2024/11/26
公開日: 2024/11/26
ジャーナル オープンアクセス HTML
電子付録In nuclear medicine, molecular imaging of the tumor microenvironment using radiopharmaceuticals (RPs) targeting cancer-associated fibroblasts is gaining significant interest. Among these RPs, [68Ga]Ga-FAPI-46 for positron emission tomography (PET) imaging is frequently used in clinical research protocols. To ensure that the production of this RP complies with good manufacturing practices, process automation is widely adopted. In this context, an automated method for preparing [68Ga]Ga-FAPI-46 was designed using a GAIA® synthesizer. Additionally, a HPLC method was developed and validated to determine the radiochemical purity (RCP) of [68Ga]Ga-FAPI-46 and ensure product quality. The validated HPLC method showed excellent repeatability, with coefficients of variation (%CV) for RCP and retention time (tR) below 0.03 and 0.16%, respectively, across 10 measurements. The radiochemical identification of [68Ga]Ga-FAPI-46 showed comparable tr values to [natGa]Ga-FAPI-46 (6.65 and 6.59 min, respectively). The limits of detection (LOD) and quantification (LOQ) were 79 and 42 kBq/mL, respectively, with a linear detector response between 62.9 and 0.08 MBq/mL (R2 = 0.9999). The method proved robust, tolerating minor variations in mobile phase flow rate and composition. This validated radio-HPLC method can be used routinely for the quality control of [68Ga]Ga-FAPI-46. Finally, three RP validation batches were produced using the automated method described and subjected to multiple quality controls. All three synthesis products met the expected specifications, notably regarding appearance, chemical and isotope identification, pH, sterility, stability, and radionuclidic and radiochemical purity.
 抄録全体を表示PDF形式でダウンロード (1926K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (1926K) HTML形式で全画面表示 -
 Mina Fukui, Yasuhiro Shimada, Kohei Tahara2024 年 72 巻 11 号 p. 1024-1033
Mina Fukui, Yasuhiro Shimada, Kohei Tahara2024 年 72 巻 11 号 p. 1024-1033
発行日: 2024/11/26
公開日: 2024/11/26
ジャーナル オープンアクセス HTML
電子付録Powders used in pharmaceuticals require good flowability. The angle of repose and compressibility index are often used to measure the flowability of pharmaceutical powders. However, confirming the relationship between external forces and flowability for smooth powder handling is necessary. Therefore, we measured pharmaceutical excipient powder using a lower cell direct movable constant-volume shear tester and evaluated the powder’s physical properties. In this study, we utilized microcrystalline cellulose, widely used as a pharmaceutical excipient and developed in many grades with different physical properties such as particle shape. We measured the shear parameters that describe the characteristic friction and cohesion properties of each microcrystalline cellulose grade. We found that the relative compression ratio (RCR) correlated with the angle of repose. Differences in the shape of the powder yield locus were observed among the grades, and the ratio of the upward convex area of the powder yield locus curve (APC) was defined as the value that quantified these differences. Furthermore, to clarify the relationship between the particle shape parameters (e.g., particle size distribution and shape) and shear parameters, we analyzed these factors using partial least squares regression. RCR was correlated with linearity and was significantly influenced by particle shape. Accurate prediction formulas were also calculated for the stress transmission and relaxation ratios. There was no correlation with the individual shape parameters, and these are considered that is involved in a complex combination. In APC, in addition to the shape parameters used in this study, bulk density had a significant effect.
 抄録全体を表示Editor's pick
抄録全体を表示Editor's pick[Highlighted Paper selected by Editor-in-Chief]
This study focused on a lower cell direct movable constant-volume shear tester that can measure flowability under stress conditions. The authors measured the shear parameters of microcrystalline cellulose, which developed in many grades with different particle shape. Differences in the shape of the powder yield locus (PYL) were observed among the grades, and the ratio of the upward convex area of the powder yield locus curve (APC) was defined as the value that quantified these differences. Furthermore, the authors examine the relationship between the particle shape parameters and shear parameters. These results are expected to improve our understanding of powder flowability.PDF形式でダウンロード (2210K) HTML形式で全画面表示
-
 Miari Kurihara, Kanaru Sasaki, Hiroki Shigehisa2024 年 72 巻 11 号 p. 1034-1037
Miari Kurihara, Kanaru Sasaki, Hiroki Shigehisa2024 年 72 巻 11 号 p. 1034-1037
発行日: 2024/11/30
公開日: 2024/11/30
ジャーナル オープンアクセス HTML
電子付録This study introduces a novel method for ring-closing chlorosulfenylation of alkenoic thioesters using N-chlorosuccinimide in hexafluoroisopropanol under mild conditions. This reaction efficiently forms five-membered cyclic sulfur compounds with high selectivity, representing a significant advancement in the synthesis of chlorinated S-heterocycles. Computational analysis using density functional theory demonstrates the superiority of thioester nucleophiles over traditional benzyl sulfides in this reaction, highlighting the energetic preference for thioesters.
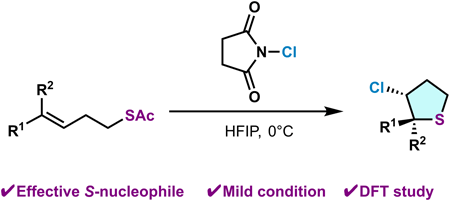 抄録全体を表示Editor's pick
抄録全体を表示Editor's pickThis study presents a novel and efficient method for the ring-closing chlorosulfenylation of alkenoic thioesters, achieved using N-chlorosuccinimide in hexafluoroisopropanol under mild conditions. This methodology enables the selective synthesis of five-membered chlorinated sulfur heterocycles with broad substrate scope. Experimental investigations highlight the superior reactivity of thioester nucleophiles compared to traditional benzyl sulfides, while density functional theory (DFT) calculations provide critical mechanistic insights. By combining synthetic practicality and mechanistic understanding, this work significantly advances sulfur chemistry and offers a versatile approach for constructing bioactive sulfur heterocycles relevant to pharmaceuticals and natural product synthesis.
PDF形式でダウンロード (990K) HTML形式で全画面表示
-
Hiroyuki Miyashita, Hitoshi Yoshimitsu2024 年 72 巻 11 号 p. 1038-1042
発行日: 2024/11/30
公開日: 2024/11/30
ジャーナル オープンアクセス HTML
電子付録A phytochemical investigation on the flesh fruits of atemoya led to the isolation of seven new kaurane type diterpenoids, (4S*,5S*,8S*,9R*,10S*,13R*,16R*)-16-hydro-18-nor-kauran-4,17-diol (1), (4S*,5S*,8S*,9R*,10S*,13R*,16S*)-18-nor-kauran-4,16,17-triol (2), (4S*,5S*,8S*,9R*,10S*,13R*,16S*)-17-acetoxy-18-nor-kauran-4,16-diol (3), (4S*,5S*,8S*,9R*,10S*,13R*,16R*)-18-nor-kauran-4,16,17-triol (4), (4S*,5S*,8S*,9R*,10S*,13R*,16S*)-17-acetoxy-16-hydro-18-nor-kauran-4-ol (5), (4R*,5S*,8S*,9R*,10S*,13R*,16S*)-16,17-dihydroxy-19-nor-kauran-4-hydroperoxide (6), and (4R*,5S*,8S*,9R*,10S*,13R*,16S*)-kauran-16,19-diol (7) along with 26 known ent-kaurane compounds. Their structures are determined on the basis of spectroscopic data and optical rotation. Compounds 1–5 were new 18-nor-kauran-4-ol type diterpenoids, which are very rarely obtained from natural sources.
 抄録全体を表示PDF形式でダウンロード (658K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (658K) HTML形式で全画面表示
- |<
- <
- 1
- >
- >|