2021 年 69 巻 7 号 p. 661-673
2021 年 69 巻 7 号 p. 661-673
In this study, based on our previous study, derivatives of naphtho[2,3-b]furan-4,9-diones were synthesized and their antimicrobial activities were evaluated. The screening of these naphthoquinones revealed that the fluorine-containing NQ008 compound exhibited potent and broad antimicrobial activities against Gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA), Gram-negative bacteria, and fungi. The results of the ratio of the minimum bactericidal concentration (MBC) to the minimum inhibitory concentrations (MICs) and time–kill assays suggest that the mode of action of NQ008 is bactericidal. Additionally, the results of a drug resistance study revealed that NQ008 exhibited potent antibacterial activity and may delay the development of bacteria resistance. Furthermore, NQ008 exhibited preliminary antiviral activity against the swine influenza virus and Feline calicivirus.
A broad variety of 1,4-naphthoquinones are found in various natural and artificial substances and are known to display a wide variety of biological activities, including antitumor, cancer chemopreventive,1,2) antimicrobial,3) antimalarial,4) trypanocidal,5) and antitubercular6) activities. Thus, 1,4-naphthoquinones are eminently useful for the development of therapeutic agents for the treatment of diseases, such as cancer and infections.7)
Among 1,4-naphthoquinones, our research group has focused on heterocycle-fused 1,4-naphthoquinones, including naphtho[2,3-b]furan-4,9-dione, 1H-benz[f]indole-4,9-dione, and naphtho[2,3-b]thiophene-4,9-dione, and has conducted synthetic and biological evaluation studies on them.8–11) Within the scope of our previous studies, we examined the antimicrobial activity of (–)-5-hydroxy-2-(1′-hydoxyethyl)naphtho[2,3-b]furan-4,9-dione, named NQ801, which is originally a constituent of Tabebuia avellanedae (Bignoniaceae), and its analogs, and found that this compound displayed modest or potent antibacterial activities against several Gram-positive bacteria, as well as antifungal activity, whereas was inactive against common Gram-negative bacteria12) (Fig. 1). These results encouraged us to explore more potent antimicrobial naphthoquinones and elucidate the specific structural requirements that induce antimicrobial activity, particularly against Gram-negative bacteria. Therefore, we further evaluated the antibacterial activities of selected naphthoquinone derivatives, which were previously synthesized in our laboratory. Additionally, fluorine-containing 1,4-naphthoquinones were synthesized and evaluated, since fluorine is conventionally incorporated into lead compounds to develop broad-spectrum antibacterial agents, represented by new quinolone (or fluoroquinolone) antibiotics, such as norfloxacin and ciprofloxacin, which are known to be effective against a broad range of Gram-positive and Gram-negative bacteria.13)

Herein, we report the synthesis and antimicrobial evaluation of a series of fluorine-containing naphthoquinone derivatives, including NQ008. Additionally, we describe the determination of the minimum bactericidal concentration (MBC), time–kill assay against Staphylococcus aureus, drug resistance study, and inhibitory activities of DNA gyrase and topoisomerase IV to obtain some insight into the mode of action of NQ008, which exhibits the highest antimicrobial activity. Moreover, we studied the effects of NQ008 against methicillin-resistant Staphylococcus aureus (MRSA) as well as its antiviral activity.
Previously, we reported concise methods for the synthesis of heterocycle-fused naphthoquinones, such as naphtho[2,3-b]-furan-4,9-dione, 1H-benz[f]indole-4,9-dione, and naphtho[2,3-b]thiophene-4,9-dione, based on the Sonogashira coupling and intramolecular cyclization reactions8,9) (Chart 1). These methods were used for the preparation of versatile heterocycle-fused naphthoquinones (NQ001–NQ014), as shown in Fig. 2. Additionally, NQ015 and NQ016, which are the phenolic derivatives of β-lapachone and lapachol, respectively, the representative antiproliferative naphthoquinones of T. avellanedae, were synthesized according to the reported method.11,14,15)


At this stage, the synthesized naphthoquinones (NQs) were tested against three Gram-positive (G+) and three Gram-negative (G−) bacteria, including Bacillus subtilis (G+, NBRC3134), Staphylococcus aureus (G+, NBRC13276), Bacillus cereus (G+, NBRC3836), Escherichia coli (G−, NBRC102203), Pseudomonas aeruginosa (G−, NBRC13275), and Salmonella enterica (G−, NBRC13245), with ciprofloxacin employed as the control. The minimum inhibitory concentrations (MICs) for these compounds are summarized in Table 1.
| Compound | MIC (µg/mL)a) | |||||
|---|---|---|---|---|---|---|
| B. subtilis NBRC3134 | S. aureus NBRC13276 | B. cereus NBRC3836 | E. coli NBRC102203 | P. aeruginosa NBRC13275 | S. enterica NBRC13245 | |
| NQ001 | >100 | >100 | >100 | >100 | >100 | >100 |
| NQ002 | 50 | 50 | 50 | >100 | >100 | >100 |
| NQ003 | >100 | 50 | >100 | >100 | >100 | >100 |
| NQ009 | 25 | 0.2 | 1.56 | >100 | >100 | >100 |
| NQ010 | 1.56 | 0.78 | 1.56 | >100 | >100 | >100 |
| NQ004 | 1.56 | 1.56 | 1.56 | 50 | >100 | >100 |
| NQ005 | 1.56 | 0.78 | 0.78 | >100 | >100 | >100 |
| NQ006 | >100 | >100 | >100 | >100 | >100 | >100 |
| NQ007 | >100 | >100 | >100 | >100 | >100 | >100 |
| NQ008 | 0.2 | 0.2 | 0.2 | 3.13 | >100 | 6.25 |
| NQ013 | 1.56 | 1.56 | 3.13 | 50 | 50 | 50 |
| NQ014 | 3.13 | 3.13 | 6.25 | >100 | 50 | >100 |
| NQ015 | 1.56 | 1.56 | 3.13 | >100 | >100 | 50 |
| NQ016 | 12.5 | 6.25 | 25 | >100 | >100 | >100 |
| NQ801 | 0.78 | 3.13 | 0.78 | >100 | >100 | >100 |
| Ciprofloxacinb) | 0.03 | 0.2 | 0.2 | 0.03 | 0.03 | 0.01 |
a) MICs were determined from the mean value of three independent experiments conducted in triplicate. b) Positive control substance.
Pyrrole-fused naphthoquinones such as NQ001–NQ003 were found to be inactive against both the G+ and G− panels, while thiophene-fused naphthoquinones such as NQ009 and NQ010 and furan-fused naphthoquinones such as NQ004–NQ008 and NQ013 and NQ014 exhibited a wide range of activities, particularly against the G+ panel. Naphthoquinones (NQ015 and NQ016), which exhibit potent antiproliferative activities against cancer cell lines, exhibited moderate antibacterial activities against the G+ panel, although their activities were slightly weaker than that of NQ801. Among the tested compounds, NQ008 exhibited the most potent activity with MICs of 0.2 and 3.13–6.25 mg/mL against three G+ bacteria and G− bacteria, except for P. aeruginosa, respectively. Notably, the MICs of NQ008 against S. aureus (G+, NBRC13276) and B. cereus (G+, NBRC3836) were comparable to those of the positive control, ciprofloxacin, and it exhibited moderate activities against two G− bacteria, although the parent NQ801 compound was inactive against two G− bacteria. Meanwhile, furan-fused naphthoquinones without oxygen functional groups at C2 such as NQ006 and NQ007 were inactive against both the G+ and the two G− bacteria panels, indicating that the substituents at C2 were crucial for the antibacterial activities.
The promising results obtained here prompted us to synthesize additional 16 compounds and further explore the antimicrobial properties of fluoride-containing furanonaphthoquinones. For the synthesis of NQ027 and NQ028, the aforementioned-synthesis method, based on the Sonogashira coupling and intramolecular cyclization reactions, was used. The halogenation of the secondary alcohol group using Deoxo-Fluor™, thionyl chloride, or phosphorus(III) bromide afforded the corresponding halogenated products, NQ022–NQ025 (Chart 2). Alternatively, NQ021 were synthesized from aforementioned NQ004.

Next, the difluorination of an unconjugated ketone, followed by manganese oxidation afforded the desired geminal difluoro products (Chart 3). Contrarily, the difluorination of a conjugated ketone under the same reaction conditions failed (Data not shown, e.g., the direct conversion of NQ005 to NQ019). Introduction of acetyl or alkoxyl moieties to NQ008 was conducted under the usual reaction conditions shown in Charts 4 and 5.



The synthesized naphthoquinones were further evaluated for their activities against three G+ and three G– bacteria. The MICs for these compounds are shown in Table 2. In general, none of the compounds exhibited a higher activity than NQ008 against the three G+ and three G− bacteria. Surprisingly, the geminal difluoro compounds (NQ017–NQ020) exhibited much lower antibacterial activities than the mono-fluorinated NQ008 compound despite their structural similarities. Furthermore, the halogen and carbon substituents on C1′ were found to be crucial for the antibacterial activities (NQ008 vs. NQ024 and NQ025). To evaluate the contribution of stereochemistry at the C1′ site to the activities, enantiomerically pure (R)-NQ008 and (S)-NQ008 were prepared by optical resolution using a chiral column (see Supplementary Materials.). As a result, the antibacterial activities of (R)-NQ008 and (S)-NQ008 against three G+ were comparable to those of the NQ008 racemate. Acetylation of phenolic hydroxyl group of NQ008 resulted in improved antibacterial activities against the three G− bacteria, notably NQ032–NQ034 showed the growth inhibitory activities against P. aeruginosa (MIC of NQ032 and NQ034: 12.5 mg/mL, MIC of NQ033: 25 mg/mL). On the other hand, alkoxylation of phenolic hydroxyl group of NQ008 significantly decreased antibacterial activities against both the three G+ and three G− bacteria (NQ038–NQ039). Next, NQ008 was chosen to be further tested against MRSA (IID1677), and it exhibited higher activity against MRSA with an MIC of 0.16 mg/mL than the positive control, vancomycin.
| Compound | MIC (µg/mL)a) | ||||||
|---|---|---|---|---|---|---|---|
| B. subtilis NBRC3134 | S. aureus NBRC13276 | B. cereus NBRC3836 | E. coli NBRC102203 | P. aeruginosa NBRC13275 | S. enterica NBRC13245 | S. aureus IID1677d) | |
| NQ008 | 0.2 | 0.2 | 0.2 | 3.13 | >100 | 6.25 | 0.16 |
| NQ017 | 1.56 | 1.56 | 0.78 | >100 | >100 | >100 | NTb) |
| NQ018 | 6.25 | 6.25 | 3.13 | >100 | >100 | >100 | NT |
| NQ019 | 3.13 | 3.13 | 50 | >100 | >100 | >100 | NT |
| NQ020 | 12.5 | 25 | 12.5 | >100 | >100 | >100 | NT |
| NQ021 | 6.25 | 3.13 | 1.56 | >100 | >100 | >100 | NT |
| NQ022 | 1.56 | 3.13 | 1.56 | >100 | >100 | >100 | NT |
| NQ023 | 0.39 | 0.39 | 0.39 | >100 | >100 | >100 | NT |
| NQ024 | 1.56 | 1.56 | 0.78 | >100 | >100 | >100 | NT |
| NQ025 | 3.13 | 6.25 | 0.39 | 100 | >100 | >100 | NT |
| NQ031 | 0.2 | 0.39 | 0.2 | 0.78 | >100 | 1.56 | NT |
| NQ032 | 0.39 | 1.56 | 0.39 | 3.13 | 12.5 | 3.13 | NT |
| NQ033 | 0.39 | 0.78 | 0.78 | 0.78 | 25 | 3.13 | NT |
| NQ034 | 0.78 | 1.56 | 0.78 | 0.78 | 12.5 | 3.13 | NT |
| NQ035 | 0.39 | 0.78 | 0.39 | 3.13 | >100 | 3.13 | NT |
| NQ038 | 50 | >100 | 50 | >100 | >100 | >100 | NT |
| NQ039 | 6.25 | 12.5 | 50 | 50 | >100 | >100 | NT |
| (R)-NQ008 | 0.2 | 0.2 | 0.2 | 3.13 | >100 | 6.25 | NT |
| (S)-NQ008 | 0.2 | 0.2 | 0.2 | 3.13 | >100 | 6.25 | NT |
| NQ801 | 0.78 | 3.13 | 0.78 | >100 | >100 | >100 | NT |
| Ciprofloxacinc) | 0.03 | 0.2 | 0.2 | 0.03 | 0.03 | 0.01 | NT |
| Vancomycinc) | 0.39 | 0.78 | 0.78 | >100 | >100 | >100 | 0.31 |
a) MICs were determined from the mean value of three independent experiments conducted in triplicate. b) NT, not tested. c) Positive control substance. d) Methicillin-resistant.
The synthesized compounds were further tested against a panel of human and plant fungal pathogens, using amphotericin B as the control. The MICs for these compounds are shown in Table 3. In this evaluation, activities similar to the antibacterial activities were observed. To our delight, NQ008 also exhibited the highest activities with MICs of 0.39–1.56 mg/mL against the tested fungi. Notably, the activities of NQ008 were comparable to those of amphotericin B against all the tested fungi. Meanwhile, the geminal difluoro products (NQ017–NQ020) were found to be inactive against the fungi panel, except against some yeasts (C. albidus or S. cerevisiae). Additionally, the halogen and carbon substituents on C1′ were found to be crucial for the antifungal activities. Exceptionally, acetylation of phenolic hydroxyl group of NQ008 drastically decreased antibacterial activities against all of human and plant fungal pathogens (The full details of the antimicrobial activities of the synthesized compounds are shown in Supplementary Materials.).
| Compound | MIC (µg/mL)a) | |||||
|---|---|---|---|---|---|---|
| C. albicans NBRC1060 | C. albidus NBRC0378 | S. cerevisiae NBRC10114 | A. fumigatus NBRC33022 | P. expansum NBRC8800 | P. variotii NBRC4855 | |
| NQ008 | 1.56 | 0.39 | 0.78 | 1.56 | 1.56 | 0.78 |
| NQ017 | >100 | >100 | 0.39 | >100 | >100 | >100 |
| NQ018 | >100 | 3.13 | 6.25 | >100 | >100 | >100 |
| NQ019 | >100 | >100 | >100 | >100 | >100 | >100 |
| NQ020 | >100 | 50 | 25 | >100 | >100 | >100 |
| NQ021 | >100 | 25 | 6.25 | >100 | 25 | 6.25 |
| NQ022 | >100 | 25 | 100 | >100 | >100 | >100 |
| NQ023 | >100 | 50 | 100 | >100 | >100 | >100 |
| NQ024 | 12.5 | 3.13 | 3.13 | 12.5 | 25 | 3.13 |
| NQ025 | 50 | 6.25 | 6.25 | 25 | 25 | 12.5 |
| NQ031 | 50 | 6.25 | 6.25 | 25 | 12.5 | 12.5 |
| NQ032 | >100 | 12.5 | 6.25 | 6.25 | 12.5 | 6.25 |
| NQ033 | >100 | >100 | >100 | >100 | 100 | >100 |
| NQ034 | >100 | >100 | 6.25 | 100 | 50 | 50 |
| NQ035 | >100 | >100 | 25 | 25 | >100 | >100 |
| NQ038 | >100 | 50 | 25 | >100 | 100 | 100 |
| NQ039 | >100 | 6.25 | 12.5 | 100 | 25 | 50 |
| NQ801 | 25 | 1.56 | 3.13 | 12.5 | 25 | 25 |
| Amphotericin Bb) | 1.56 | 1.56 | 0.78 | 1.56 | 1.56 | 0.78 |
a) MICs were determined from the mean value of three independent experiments conducted in triplicate. b) Positive control substance.
The MBC of NQ008 was evaluated, and the ratio of MBC to MIC was calculated to determine the bactericidal vs. bacteriostatic mode of action of the compounds. NQ008 showed an MBC of 0.2 mg/mL against S. aureus NBRC13276 with an MBC to MIC ratio of 1, which suggests a bactericidal mode of action since compounds are usually regarded as bactericidal if the MBC is less than four times the MIC.
Time–Kill AssayTime–kill kinetic assays were performed to determine the bactericidal (99.9% kill or a ≥3log10 colony forming unit (CFU)/mL reduction in colony count from the initial inoculum) or bacteriostatic (<3log10 CFU/mL reduction) nature of the compounds.16,17) NQ008 was subjected to a time–kill analysis against S. aureus NBRC 13276 to determine the kinetics of its kills. As shown in Fig. 3, the bacterial cells grew rapidly in the first 8 h without NQ008. Subsequently, the growth of S. aureus went into the stationary phase after 8 h of incubation. Contrary, 16 × MIC of NQ008 rapidly reduced the viable counts (>103 CFU mL−1) of S. aureus after incubation for 4 h. NQ008 exhibited more bactericidal tendencies against S. aureus NBRC 13276 and more rapid bactericidal kinetics than vancomycin, which was used as the control. The data suggest a bactericidal mode of action that is consistent with the MBC to MIC ratio described above.

Results are the average of at least three replicate experiments, and error bars show the standard error of the mean. (Color figure can be accessed in the online version.)
To further evaluate the resistance to NQ008, we selected two commercial antibiotics (rifampin and norfloxacin) as positive controls against S. aureus NBRC 13276. As shown in Fig. 4, no change in the MIC of NQ008 was observed, while those of the positive controls, rifampin and norfloxacin, increased by over 20- and 90-fold, respectively, after 20 passages. The results showed that NQ008 exhibits potent antibacterial activity, and it delays the development of bacteria resistance toward S. aureus.

(Color figure can be accessed in the online version.)
The inhibition of DNA gyrase or topoisomerase IV activity is known to be one of the mechanisms of action of antimicrobials.18) We examined the inhibitory activity of NQ801, NQ008 and NQ031 on DNA gyrase and topoisomerase IV (Figs. 5, 6). Novobiocin, which is a currently available DNA gyrase inhibitor, inhibited supercoiling activity in a concentration-dependent manner and completely inhibited supercoiling activity at 3 µM (Fig. 5). On the other hand, NQ801 and NQ008 had no inhibitory activity against DNA gyrase at 300 µM and NQ031 exhibited faint inhibitory activity at 300 µM (Fig. 5). In the decatenation assay of topoisomerase IV, ciprofloxacin, which was a well-known quinolone antibiotic and topoisomerase IV inhibitor, was used as the positive control and inhibited 70% of the activity of topoisomerase IV at 50 µM. In this evaluation, it was found that acetylation of phenolic hydroxyl group of NQ008 induces weak inhibitory activities toward topoisomerase IV although NQ801 and NQ008 had no inhibitory activity against topoisomerase IV at 300 µM. These results indicated that the targets of this compound were neither topoisomerase IV nor DNA gyrase.

DNA gyrase and relaxed plasmid DNA were added to the reaction mixture and incubated at 37 °C for 30 min. The mixture was electrophoresed on a 1% agarose gel and stained by ethidium bromide.
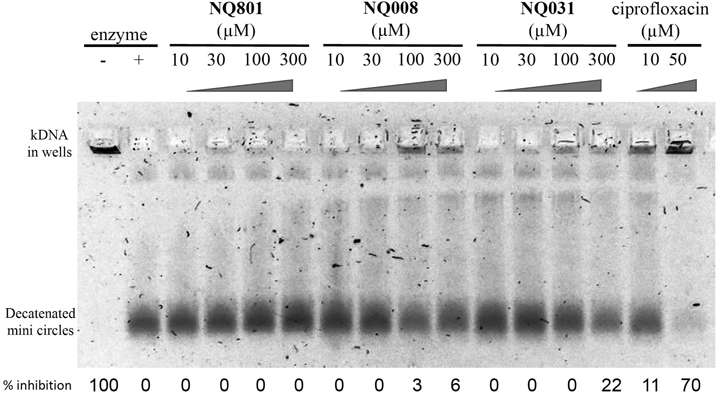
Topoisomerase IV and kinetoplast DNA were added to the reaction mixture. The mixture was electrophoresed on a 1% agarose gel and stained by ethidium bromide.
To further extensively evaluate the biological properties, NQ008 was subjected to a cell-based antiviral assay against swine influenza virus A/IOWA/15/30 (H1N1) and Feline calicivirus F9 (FCV/F9). Influenza remains seriously detrimental to public health because of the appearance of resistant strains. Meanwhile, FCV/F9, which belongs to the vesivirus genus in the Caliciviridae family, is usually used as the surrogate for human norovirus because human noroviruses are not cultivable. The infectivity titers of the swine influenza virus and FCV/F9 were determined as the 50% tissue culture infectious dose, (TICD50)/0.1 mL according to the Spearman Kärber method. The preliminary antiviral activity revealed that NQ008 inhibited the infection of Madin–Darby canine kidney (MDCK) cells by the swine influenza virus (35% decrease in TICD50/0.1 mL at 3.0 µg/mL) and Crandell Rees feline kidney (CRFK) cells by FCV/F9 (60% decrease in TICD50/0.1 mL at 3.0 µg/mL). The details of the antiviral activity are shown in Supplementary Data. In our laboratory, further study on the antiviral activity of NQ008 is underway and the results will be reported in due course.19)
In conclusion, novel fluorine-containing furanonaphthoquinones were synthesized, and their antimicrobial activities were evaluated. Among the synthesized compounds, NQ008 exhibited the most potent and broad antimicrobial activities against Gram-positive bacteria including MRSA, Gram-negative bacteria, and fungi. Notably, the mono-fluorinated substituents on the furan ring were crucial for the improvement of the antimicrobial activity. Moreover, acetylation of phenolic hydroxyl group of NQ008 resulted in improved antibacterial activities against the three G− bacteria. In the time–kill assays, NQ008 exhibited rapid bactericidal activity against S. aureus at a relatively high concentration. The results of the drug resistance study revealed that NQ008 delays the development of bacterial resistance. Based on these results, it may be beneficial to conduct further structural modification studies and in-depth antibacterial research on the developed fluorine-containing furanonaphthoquinones.
All melting points were taken on a Yanagimoto micromelting point apparatus and were uncorrected. 1H-, 13C- and 19F-NMR spectra were acquired with Bruker-Biospin Avance III 400 MHz NMR spectrometer and taken in CDCl3, unless otherwise noted. Chemical shift values are expressed in ppm relative to internal tetramethylsilane. Coupling constants J values are presented in Hz. Abbreviations are as follows: s, singlet; d, doublet; t, triplet; m, multiplet. IR spectra were recorded with a Shimadzu IRAffinty-1S spectrometer. IR spectroscopy of oil sample was measured as neat liquid film. The wave-numbers of maximum absorption peaks of IR spectroscopy are presented in cm−1. MS (electrospray ionization (ESI)) is presented in m/z. Extracts were washed with brine and then dried over sodium sulfate. Silica gel column chromatography was used for purification. Compound 1 was prepared according to a known literature procedure.
Antibacterial MIC DeterminationA solid culture of a selected bacterial strain was inoculated into Mueller–Hinton broth added 1.5% agar at 37 °C for 18 h. The bacteria concentration (McFarland Standard No. 0.5 of 1.5 × 108 CFU/mL) was determined and diluted with 0.85% Physiological saline. The bacterial culture was transferred to fresh MH broth and diluted to a final concentration of approximately 1.5 × 106 CFU/mL in a 96-well microplate. Then, a series of solutions (0.1 mL each in 2-fold dilution) of the tested compounds was added to the testing wells. The MIC is defined as the minimum concentration of compound needed to completely inhibit the growth of bacteria. After 18 h of incubation at 37 °C, The MIC value is defined as the lowest concentration of test compounds which prevents visible growth of bacterium. The MIC determinations for each experiment were repeated at least three times.
Determination of Minimum Bactericidal ConcentrationTo determine the MBC, 10 µL was taken from all wells of the MIC plate at and above the MIC and spotted onto Mueller–Hinton broth added 1.5% agar. The agar plates were incubated at 37 °C for 18 h and the MBC was defined as the concentration at which no colonies were seen. The ratio MBC/MIC that was used to evaluate if the compound is bactericidal (MBC/MIC = 1 or 2) or bacteriostatic (MBC/MIC = 4 or 16).
Antifungal MIC DeterminationsA solid culture of a selected fungal strain was inoculated into Difco™ Potato Dextrose agar at 28 °C for 3 or 5 d. The fungal concentration (1.0–2.0 × 106 CFU/mL) was determined and diluted with 0.05% Tween80 solution. The fungal culture was transferred to fresh PD broth and diluted to a final concentration of approximately 1.0–2.0 × 105 CFU/mL in a 24-well microplate. Then, a series of solutions (1 mL each in 2-fold dilution) of the tested compounds was added to the testing wells. The MIC is defined as the minimum concentration of compound needed to completely inhibit the growth of fungus. After 3 or 5 d of incubation at 28 °C, The MIC value is defined as the lowest concentration of test compounds which prevents visible growth of bacterium. The MIC determinations for each experiment were repeated at least three times.
Time–Kill CurvesTime–kill curve analyses were performed by culturing the S. aureus NBRC 13276 in the presence of VCM and NQ008. Flasks of MHB were inoculated with test organism at a concentration 5 × 106 CFU/mL in a total volume of 50 mL. The antimicrobial agents were then added at a concentration of 16 × MIC and incubated at 37 °C in a shaking incubator at 110 rpm. Samples (0.10 mL) were taken for determination of viable counts at 0, 2, 4, 6 h and at 24 h after the substances had been added. Serial dilutions were prepared in 0.85% physiological saline and were plated onto Mueller–Hinton agar plates. Plates were incubated at 37 °C for 18 h and the number of colonies was then determined. Kill curves were constructed by plotting the log 10 CFU/mL over 24 h. Time–kill curves were repeated at least three times.
Drug Resistance StudyThe drug resistance study was based on the MICs tests. The initial MIC values of a compound and two control antibiotics (rifampin and norfloxacin) against S. aureus NBRC 13276 were determined as described above. The bacterial culture from 96-well microtiter plate at a concentration of 0.5 × MIC was continuously used to prepare the bacterial dilution (approximately 1.5 × 106 CFU/mL) for the next MIC assay. Then these bacterial suspensions were seeded into another new 96-well microtiter plate and incubated with various concentrations of a compound and the two control antibiotics. After incubation for 24 h at 37 °C, the fold change of MIC values was determined. This process was repeated for about 20 passages.
DNA Gyrase Supercoiling Assay and Topoisomerase IV Decatenation AssayThe S. aureus gyrase supercoiling assay kit (Inspiralis Co., Ltd., U.K.) was used to investigate the effects of DNA gyrase. One unit of DNA gyrase converted 500 ng relaxed pBR322 to the supercoiled form in 30 min. The enzyme and substrate DNA were incubated at 37 °C in reaction mixture (40 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES)-KOH (pH 7.6), 10 mM magnesiumacetate, 500 mM potassium glutamate, 10 mM dithiothreitol (DTT), 2 mM ATP, and 50 µg/mL albumin) for 30 min.
The S. aureus topoisomerase IV decatenation assay kit (Inspiralis Co., Ltd.) was used for DNA topoisomerase IV. One unit of DNA topoisomerase IV decatenated 200 ng of kinetoplast DNA to mini-circle DNA in 30 min. The enzyme and substrate DNAwere incubated at 37 °C in reaction mixture (50 mM Tris–HCl (pH 7.5), 5 mM magnesium chloride, 5 mM DTT, 1.5 mM ATP, 350 mM potassium glutamate, and 50 µg/mL albumin) for 30 min.
The reaction mixture following incubation was electrophoresed in 1% agarose gels at 50 V for 1.5 h for decatenation assay and 3 h for supercoiling assay, respectively. Gels were stained with 1 µg/mL ethidium bromide for 20 min and washed with distilled water for 15 min.
The density of the specific bands was quantified employing the software ImageJ (NIH, Bethesda, MD, U.S.A.).
Antiviral ActivityThe inactivation of swine influenza virus and Feline calicivirus with NQ008 was determined as previously described.20)
Synthesis2-(1-Hydroxyethyl)-1-methyl-1H-benzo[f]indole-4,9-dione (NQ001)8)1H-NMR (CDCl3) δ: 1.69 (3H, d, J = 6.5 Hz), 2.00 (1H, d, J = 7.0 Hz), 4.12 (3H, s), 4.94 (1H, dq, J = 6.5, 7.0 Hz), 6.69 (1H, s), 7.65–7.69 (2H, m), 8.11–8.14 (2H, m).
1-(4,9-Dioxo-4,9-dihydro-1H-benzo[f]indol-2-yl)ethyl Acetate (NQ002)10)1H-NMR (CDCl3) δ: 1.70 (3H, d, J = 6.5 Hz), 2.12 (3H, s), 5.95 (1H, dq, J = 2.0, 6.5 Hz), 6.78 (1H, d, J = 2.0 Hz), 7.67–7.72 (2H, m), 8.14–8.20 (2H, m), 9.93 (1H, br s).
5-Hydroxy-2-(1-hydroxyethyl)-1-methyl-1H-benzo[f]indole-4,9-dione (NQ003)9)1H-NMR (CDCl3) δ: 1.69 (3H, d, J = 6.5 Hz), 4.13 (3H, s), 4.95 (1H, q, J = 6.5 Hz), 6.68 (1H, s), 7.18 (1H, dd, J = 1.1, 8.4 Hz), 7.54 (1H, dd, J = 7.3, 8.4 Hz), 7.65 (1H, dd, J = 1.1, 7.3 Hz), 12.58 (1H, s).
2-(1-Hydroxyethyl)naphtho[2,3-b]furan-4,9-dione (NQ004)9)1H-NMR (CDCl3) δ: 1.59 (d, 3H, J = 6.7 Hz), 4.98 (1H, q, J = 6.7 Hz), 6.79 (1H, s), 7.66–7.71 (2H, m), 8.10–8.16 (2H, m).
2-Acetyl-naphtho[2,3-b]furan-4,9-dione (NQ005)21)To a solution of NQ004 (40 mg, 0.13 mmol) in CHCl3 (10 mL) was added MnO2 (115 mg, 1.3 mmol). After stirred for 6 h at room temperature (r.t.), the mixture was filtered through a pad of Celite. The Celite was washed with CHCl3, and the combined organics were concentrated. The crude product was chromatographed on silica gel. Yield 78% (30 mg). Orange solid. Rf (hexane/EtOAc = 2/1) = 0.50. 1H-NMR (CDCl3) δ: 2.65 (3H, s), 7.59 (1H, s), 7.79 (2H, m), 8.23 (2H, m).
5-Hydroxy-2-phenylnaphtho[2,3-b]furan-4,9-dione (NQ006)9)1H-NMR (CDCl3) δ: 7.36 (1H, dd, J = 1.2, 8.5 Hz), 7.42–7.48 (3H, m), 7.57 (1H, s), 7.60–7.62 (1H, m), 7.88 (1H, dd, J = 1.2, 7.5 Hz), 7.91–7.94 (2H, m), 12.55 (1H, s).
5-Hydroxy-2-phenethylnaphtho[2,3-b]furan-4,9-dione (NQ007)9)1H-NMR (CDCl3) δ: 3.06–3.16 (4H, m), 6.54 (1H, s), 7.19–7.32 (6H, m), 7.60 (1H, dd, J = 7.4, 8.3 Hz), 7.74 (1H, dd, J = 1.1, 7.4 Hz), 12.17 (1H, s).
2-(1-Fluoroethyl)-5-hydroxynaphtho[2,3-b]furan-4,9-dione (NQ008)A solution of NQ8019) (100 mg, 0.39 mmol) in dichloromethane (5.00 mL) was added dropwise to Deoxo Fluor (82.0 µL, 0.47 mmol) at 0 °C. After stirred for 3 h at r.t., the mixture was treated with H2O, and extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ008 (79 mg, 78%) as a yellow solid. mp 149–151 °C. Rf (hexane/EtOAc = 2/1) = 0.46.
1H-NMR (CDCl3) δ: 1.80 (dd, J = 6.7 Hz, 23.2 Hz, 3H), 5.75 (dq, J = 6.6 Hz, 13.1 Hz, 47.6 Hz, 1H), 6.98 (d, J = 2.6 Hz, 1H), 7.30 (dd, J = 1.2 Hz, 8.5 Hz, 1H), 7.64 (dd, J = 8.5 Hz, 7.5 Hz, 1H), 7.78 (dd, J = 1.2 Hz, 7.5 Hz, 1H), 12.17 (s, 1H).
13C-NMR (CDCl3) δ: 19.2 (d, J = 23.9 Hz, CH3), 83.1 (d, J = 168.9 Hz, CH), 105.7 (d, J = 4.4 Hz, CH), 115.2 (C), 120.1 (CH), 125.4 (CH), 130.6 (d, J = 1.7 Hz, C), 132.6 (C), 136.4 (CH), 152.5 (d, J = 1.6 Hz, C), 160.0 (d, J = 24.1 Hz, C), 162.4 (C), 172.7 (C), 186.2 (C).
19F-NMR (CDCl3): δ –169.6 (s, 1F).
IR (KBr): 704.02, 831.32, 1035.77, 1228.66, 1319.3, 1336.7, 1450.5, 1539.2, 1641.4, 1674.2.
High resolution (HR)MS (ESI) m/z: [M + Na]+ Calcd for [C14H9FO4Na]+, 267.0433. Found, 267.0439.
2-Acetyl-5-hydroxynaphtho[2,3-b]thiophene-4,9-dione (NQ009)To a solution of NQ010 (26 mg, 0.14 mmol) in CHCl3 (10 mL) was added MnO2 (380 mg, 4.4 mmol). After stirred for 1 h at r.t., the mixture was filtered through a pad of Celite. The Celite was washed with CHCl3, and the combined organics were concentrated. The crude product was chromatographed on silica gel. Yield 69% (18 mg). yellow solid. mp 258–260 °C. Rf (hexane/EtOAc = 2/1) = 0.40.
1H-NMR δ: 2.69 (s, 3H), 7.33 (d, J = 7.5 Hz, 1H), 7.67 (dd, 1H, J = 7.5, 7.5 Hz), 7.81 (7.33 (d, J = 7.5 Hz, 1H), 8.16 (s, 1H), 12.3 (s, 1H).
13C-NMR (CHCl3) δ: 27.0 (CH3), 115.7 (C), 120.4 (CH), 125.4 (CH), 129.2 (CH), 133.6 (C), 136.6 (CH), 142.2 (C), 150.0 (C), 150.7 (C), 162.9 (C), 177.6 (C), 184.5 (C), 190.6 (C).
IR (KBr): 594, 756, 802, 1026, 1095, 1219, 1257, 1296, 1358, 1450, 1628, 1659, 3086.
HRMS (ESI) m/z: [M − H]− Calcd for [C14H7O4S]−, 271.0071. Found, 271.0059.
5-Hydroxy-2-(1-hydroxyethyl)naphtho[2,3-b]thiophene-4,9-dione (NQ010)9)1H-NMR (CDCl3–MeOD) δ: 1.62 (3H, d, J = 6.5 Hz), 5.14 (1H, q, J = 6.5 Hz), 7.26 (1H, dd, J = 1.0, 8.3 Hz), 7.47 (1H, s), 7.62 (1H, dd, J = 7.5, 8.3 Hz), 7.74 (1H, dd, J = 1.0, 7.5 Hz), 12.33 (1H, s).
2-Acetyl-6-hydroxynaphtho[1,2-b]furan-4,5-dione (NQ013)11)1H-NMR δ: 1.62 (d, 3H, J = 6.6 Hz), 2.08 (s, 1H), 4.94 (q, 1H, J = 6.6 Hz), 6.69 (s, 1H), 7.00 (d, 1H, J = 8.8 Hz), 7.25 (d, 1H, J = 7.3 Hz), 7.52 (dd, 1H, J = 7.3, 8.8 Hz), 11.98 (s, 1H).
2-Acetyl-6-hydroxynaphtho[1,2-b]furan-4,5-dione (NQ014)11)1H-NMR δ: 2.58 (s, 3H), 7.15 (d, J = 8.6 Hz, 1H), 7.50 (d, J = 7.4 Hz, 1H), 7.52 (s, 1H), 7.64 (dd, J = 7.5, 8.6 Hz, 1H), 12.02 (s, 1H).
7-Hydroxy-2,2-dimethyl-3,4-dihydro-2H-benzo[h]chromene-5,6-dione (NQ015)11)1H-NMR δ: 1.45 (s, 6H), 1.83 (t, J = 6.9 Hz, 2H), 2.55 (t, J = 6.9 Hz, 2H), 7.05 (d, J = 8.5 Hz, 1H), 7.34 (d, J = 7.6 Hz, 1H), 7.52 (dd, J = 7.6, 8.5 Hz, 1H), 11.99 (s, 1H).
2,5-Dihydroxy-3-(3-methylbut-2-enyl)naphthalene-1,4-dione (NQ016)14)1H-NMR (CDCl3) δ: 1.70 (s, 3H), 1.78 (s, 3H), 3.28 (d, J = 7.2 Hz, 2H), 5.20 (t, J = 7.2 Hz, 1H), 7.28 (dd, J = 1.6, 6.7 Hz, 1H), 7.42 (s, 1H), 7.53 (t, J = 7.6 Hz, 1H), 7.63 (dd, J = 1.6, 7.6 Hz, 1H), 12.47 (s, 1H).
2-(1,1-Difluoroethyl)-5-hydroxynaphtho[2,3-b]furan-4,9-dione (NQ017)Under Ar atmosphere, a solution of NQ018 (168 mg, 0.60 mmol) in toluene (20.0 mL) was heated with MnO2 (1.30 g, <10 µm, activated, 15.0 mmol) to reflux for 3 h. After cooled to r.t., the mixture was filtered through a pad of Celite, and then concentrated. The residue was passed through a silica gel column chromatography (CHCl3). Concentration gave NQ017 (50 mg, 30% yield) as yellow solid. mp 164–166 °C. Rf (hexane/EtOAc = 5/1) = 0.23.
1H-NMR (CDCl3) δ: 2.09 (t, J = 18.4 Hz, 3H), 7.14 (t, J = 1.2 Hz, 1H), 7.30 (dd, J = 1.2 Hz, 8.5 Hz, 1H), 7.65 (dd, J = 7.5 Hz, 8.5 Hz, 1H), 7.78 (dd, J = 1.2 Hz, 7.5 Hz, 1H), 12.11 (s, 1H).
13C-NMR (CDCl3) δ: 23.2 (t, J = 26.9 Hz, CH3), 106.2 (t, J = 3.1 Hz, CH), 115.2 (C), 116.1 (t, J = 237.1 Hz, C), 120.3 (CH), 125.7 (CH), 130.2 (C), 132.5 (C), 136.5 (CH), 152.7 (C), 155.2 (t, J = 37.9 Hz, C), 162.5 (C), 172.7(C), 185.8 (C).
19F-NMR (CDCl3): δ −89.0 (s, 2F).
IR (KBr): 756, 1229, 1277, 1641, 1670.
HRMS (ESI) m/z: [M + H]+ Calcd for [C14H9O4F2]+, 279.0469. Found, 279.0457
2-(1,1-Difluoroethyl)-5-hydroxy-2,3-dihydronaphtho[2,3-b]furan-4,9-dione (NQ018)A solution of NQ02912) (200 mg, 0.78 mmol) in dichloromethane (8 mL) was added dropwise to Deoxo Fluor (408 µL, 2.33 mmol) at 0 °C. After stirred for 3 h at r.t., the mixture was treated with H2O, and extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ018 (175 mg, 80%) as a yellow solid. mp 159–161 °C. Rf (hexane/EtOAc = 2/1) = 0.5.
1H-NMR (CDCl3) δ: 1.81 (t, J = 18.9 Hz, 3H), 3.26–3.40 (m, 2H), 4.98–5.08 (m, 1H), 7.28 (dd, J = 1.1 Hz, 8.4 Hz, 1H), 7.56 (dd, J = 7.5 Hz, 8.4 Hz, 1H), 7.64 (dd, J = 1.1 Hz, 7.5 Hz, 1H), 12.11 (s, 1H).
13C-NMR (CDCl3) δ: 20.2 (dd, J = 25.8 Hz, 25.6 Hz, CH3), 27.2 (dd, J = 2.5 Hz, 4.0 Hz, CH2), 84.4 (dd, J = 29.1 Hz, 37.4 Hz, CH), 114.7 (C), 119.6 (CH), 120.7 (dd, J = 239.1 Hz, 245.4 Hz, C), 123.9 (C), 125.9 (CH), 131.7 (C), 135.4 (CH), 160.2 (C), 161.3 (C), 176.2 (C), 187.6 (C).
19F-NMR (CDCl3) δ: −109.9 (d, J = 257.7 Hz, 1F), −101.9 (d, J = 257.7 Hz, 1F).
IR (KBr): 758, 1037, 1159, 1219, 1238, 1340, 1452, 1618, 1651, 1681.
HRMS (ESI) m/z: [M + H]+ Calcd for [C14H11O4F2]+, 281.0625. Found, 281.0617.
2-(1,1-Difluoroethyl)naphtho[2,3-b]furan-4,9-dione (NQ019)Under Ar atmosphere, a solution of NQ020 (39.8 mg, 0.15 mmol) in toluene (3.00 mL) was heated with MnO2 (328 mg, <10 µm, activated, 3.77 mmol) to reflux for 3 h. After cooled to r.t., the mixture was filtered through a pad of Celite, and then concentrated. The residue was passed through a silica gel column chromatography (CHCl3). Concentration gave NQ019 (24 mg, 60% yield) as yellow solid. mp 190–192 °C. Rf (hexane/EtOAc = 2/1) = 0.5.
1H-NMR (CDCl3) δ: 2.09 (t, J = 18.3 Hz, 3H), 7.16 (t, J = 1.2 Hz, 1H), 7.78–7.80 (m, 2H), 8.20–8.26 (m, 2H)
13C-NMR (CDCl3) δ: 23.2 (t, J = 27.0 Hz, CH3), 106.5 (t, J = 3.2 Hz, CH), 116.2 (t, J = 236.7 Hz, C), 127.1 (CH), 127.2 (CH), 130.4 (C), 132.3 (C), 133.0 (C), 134.2 (CH), 134.2 (CH), 152.6 (C), 155.0 (t, J = 37.6 Hz, C), 173.5 (C), 178.0 (C).
19F-NMR (CDCl3) δ: −89.0(s, 2F)
IR (KBr): 418.55, 717.52, 894.97, 964.41, 1128.36, 1188.15, 1234.44, 1273.02, 1290.38, 1369.46, 1593.20, 1681.93 cm−1
HRMS (ESI) m/z: [M + Na]+ Calcd for [C14H8F2O3Na]+, 285.0339. Found, 285.0347.
2-(1,1-Difluoroethyl)-2,3-dihydronaphtho[2,3-b]furan-4,9-dione (NQ020)A solution of NQ03021) (200 mg, 0.83 mmol) in dichloromethane (8.00 mL) was added dropwise to Deoxo Fluor (435 µL, 2.48 mmol) at 0 °C. After stirred for 3 h at r.t., the mixture was treated with H2O, and extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ020 (176 mg, 81%) as a yellow solid. mp 134–136 °C. Rf (hexane/EtOAc = 2/1) = 0.42.
1H-NMR (CDCl3) δ: 1.81 (t, J = 18.9 Hz, 3H), 3.28–3.41 (m, 2H), 5.00–5.06 (m, 1H), 7.70 (ddd, J = 1.6, 7.5, 14.2 Hz, 1H), 7.75 (ddd, J = 1.6, 7.5, 14.2 Hz, 1H), 8.08 (dd, J = 1.5, 4.8 Hz, 1H), 8.10 (dd, J = 1.5, 4.8 Hz, 1H).
13C-NMR (CDCl3) δ: 20.2 (dd, J = 25.8, 52.1 Hz, CH3), 27.7 (dd, J = 2.2, 4.5 Hz, CH2), 84.1 (dd, J = 29.2, 37.2 Hz, CH), 120.9 (dd, J = 239.8, 245.5 Hz, C), 124.3 (C), 126.2 (CH), 126.4 (CH), 131.5 (C), 132.8 (C), 133.2 (CH), 134.3 (CH), 159.4 (C), 177.0 (C), 181.8 (C).
19F-NMR (CDCl3) δ: −109.7 (d, J = 256.1 Hz, 1F), −101.8 (d, J = 256.1 Hz, 1F)
IR (KBr): 419, 723, 916, 990, 1113, 1163, 1196, 1207, 1246, 1294, 1358, 1387, 1634, 1657, 1684.
HRMS (ESI) m/z: [M + Na]+ Calcd for [C14H10O3F2Na]+, 287.0496. Found, 287.0503.
2-(1-Fluoroethyl)naphtho[2,3-b]furan-4,9-dione (NQ021)A solution of NQ004 (50.0 mg, 0.20 mmol) in dichloromethane (4.00 mL) was added dropwise to Deoxo Fluor (43.5 µL, 0.25 mmol) at 0 °C. After stirred for 3 h at r.t., the mixture was treated with H2O, and extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ021 (36 mg, 74%) as a yellow solid. mp 158–160 °C. Rf (hexane/EtOAc = 4/1) = 0.33.
1H-NMR (CDCl3) δ: 1.31 (dd, J = 6.6, 23.2 Hz, 3H), 5.73 (dq J = 6.7, 47.7 Hz, 1H), 6.99 (d, J = 2.6 Hz, 1H), 7.75–7.78 (m, 2H), 8.18–8.24 (m, 2H).
13C-NMR (CDCl3) δ: 19.2 (d, J = 24.1 Hz, CH3), 83.2 (d, J = 168.7 Hz, CH), 106.1 (d, J = 4.7 Hz, CH), 127.0 (CH), 127.1 (CH), 130.9 (C), 132.4 (C), 133.0 (C), 134.0 (CH), 134.0 (CH), 152.4 (C), 159.8 (d, J = 24.6 Hz, C), 173.5 (C), 180.3 (C).
19F-NMR (CDCl3) δ: −169.2 (s, 1F).
IR (KBr): 718, 843, 943, 957, 1065, 1198, 1221, 1341, 1541, 1593, 1672.
HRMS (ESI) m/z: [M + Na]+ Calcd for [C14H9O3F1Na]+, 267.0433. Found, 267.0439.
5-Hydroxy-2-(1-hydroxybutyl)naphtho[2,3-b]furan-4,9-dione (NQ027)Under Ar atmosphere, a mixture of Cu2O (45.2 mg, 0.30 mmol), 1-phenyl-2-propyn-1-ol (77.0 µL, 0.60 mmol), NQ02622) (100 mg, 0.30 mmol) and Pd(OAc)2 (2.20 mg, 0.01 mmol) in N,N-dimethylformamide (DMF) (5.00 mL) and pyridine (4.00 mL, 50.0 mmol) was stirred for 1 h at 80 °C. The mixture was filtered through a pad of Celite. The Celite was washed with EtOAc, and the combined organics were concentrated. The crude product was diluted with CHCl3. The organic phase were washed with H2O and brine, dried over Na2SO4, and then concentrated. The crude product was chromatographed on silica gel. Yield 48% (49 mg). mp 168–170 °C. yellow solid. Rf (hexane/EtOAc = 1/1) = 0.25.
1H-NMR (CDCl3) δ: 2.80 (d, J = 4.23 Hz, 1H), 5.95 (d, J = 3.65 Hz, 1H), 6.74 (d, J = 0.82 Hz 1H), 7.25 (dd, J = 1.19, 8.31 Hz, 1H), 7.35–7.49 (m, 5H), 7.60 (dd, J = 7.64, 8.32 Hz, 1H), 7.73 (dd, J = 1.17, 7.46 Hz, 1H), 12.14 (s, 1H).
13C-NMR (CDCl3) δ: 70.3 (CH), 105.2 (CH), 115.2 (C), 120.0 (CH), 125.3 (CH), 126.6 (CH), 126.7(CH), 129.0 (CH), 130.9 (C), 132.7 (C), 136.3 (CH), 139.0 (C), 152.4 (C), 162.4 (C), 163.8 (C), 172.7 (C), 186.5 (C).
IR (KBr): 698, 733, 748, 1030, 1061, 1161, 1223, 1292, 1346, 1377, 1451, 1535, 1597, 1643, 1667, 3460.
HRMS (ESI) m/z: [M + Na]+ Calcd for [C19H12O5Na]+, 343.0582. Found, 343.0577.
2-(Fluoro(phenyl)methyl)-5-hydroxynaphtho[2,3-b]furan-4,9-dione (NQ022)A solution of NQ027 (48.6 mg, 0.15 mmol) in dichloromethane (4.00 mL) was added dropwise to Deoxo Fluor (32.0 µL, 0.18 mmol) at 0 °C. After stirred for 0.5 h at r.t., the mixture was treated with H2O, and extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ022 (43 mg, 90%) as a yellow solid. mp 160–162 °C. Rf (hexane/EtOAc = 3/1) = 0.43.
1H-NMR (CDCl3) δ: 6.53 (d, J = 46.8 Hz, 1H), 6.80 (d, J = 2.47 Hz, 1H), 7.27 (dd, J = 1.14, 8.40 Hz, 1H), 7.49–7.45 (m, 5H), 7.62 (dd, J = 7.52, 8.40 Hz, 1H), 7.76 (dd, J = 1.14, 7.52 Hz), 12.12 (s, 1H).
13C-NMR (CDCl3) δ: 87.1 (d, J = 173.8 Hz, CH), 107.5 (d, J = 4.15 Hz, CH), 115.1 (C), 120.2 (CH), 125.5 (CH), 126.5 (d, J = 6.0 Hz, CH), 129.0 (CH), 129.8 (d, J = 1.68, CH), 130.5 (C), 132.6 (C), 135.1 (d, J = 21.9 Hz, C), 136.4 (CH), 153.0 (C), 159.2 (d, J = 27.4 Hz, C), 162.4 (C), 172.8 (C), 186.1 (C).
19F-NMR (CDCl3) δ: −169.7 (s, 1F).
IR (KBr): 702, 752, 829, 1018, 1038, 1065, 1161, 1177, 1227, 1292, 1308, 1323, 1350, 1377, 1454, 1493, 1597, 1643, 1674, 3132.
HRMS (ESI) m/z: [M − H]− Calcd for [C19H10O4F]−, 321.0563. Found, 321.0570.
5-Hydroxy-2-(1-hydroxybutyl)naphtho[2,3-b]furan-4,9-dione (NQ028)Under Ar atmosphere, a mixture of Cu2O (45.2 mg, 0.30 mmol), 1-hexyn-3-ol (70.6 µL, 0.60 mmol), NQ02622) (100 mg, 0.30 mmol) and Pd(OAc)2 (2.20 mg, 0.01 mmol) in DMF (5.00 mL) and pyridine (4.00 mL, 50.0 mmol) was stirred for 1 h at 80 °C. The mixture was filtered through a pad of Celite. The Celite was washed with EtOAc, and the combined organics were concentrated. The crude product was diluted with CHCl3. The organic phase were washed with H2O and brine, dried over Na2SO4, and then concentrated. The crude product was chromatographed on silica gel. Yield 60% (54.1 mg). Yellow solid. mp 113–115 °C. Rf (hexane/EtOAc = 1/1) = 0.6.
1H-NMR (CDCl3) δ: 0.99 (t, J = 7.41 Hz, 3H), 1.39–1.58 (m, 2H), 1.85–1.99 (m, 2H), 2.28 (s, 1H), 4.87 (dd, J = 5.46, 7.40 Hz, 1H), 6.83 (s, 1H), 7.26 (dd, J = 0.99, 8.32 Hz, 1H), 7.60 (dd, J = 7.74, 8.32 Hz, 1H), 7.75 (dd, J = 0.99, 7.74 Hz, 1H), 12.16 (s, 1H).
13C-NMR (CDCl3) δ: 13.8 (CH3), 18.5 (CH2), 37.6 (CH2), 67.3 (CH), 103.9 (CH), 115.2 (C), 120.0 (CH), 125.3 (CH), 131.0 (C), 132.7 (C), 136.3 (CH), 152.0 (C), 162.3 (C), 165.1 (C), 172.7 (C), 186.5 (C).
IR (KBr): 756, 1034, 1231, 1381, 1454, 1539, 1578, 1597, 1640, 1670, 3402.
HRMS (ESI) m/z: [M + H]+ Calcd for [C16H15O5]+, 287.0919. Found, 287.0914.
2-(1-Fluorobutyl)-5-hydroxynaphtho[2,3-b]furan-4,9-dione (NQ023)A solution of NQ028 (20.0 mg, 0.07 mmol) in dichloromethane (4.00 mL) was added dropwise to Deoxo Fluor (18.4 µL, 0.10 mmol) at 0 °C. After stirred for 0.5 h at r.t., the mixture was treated with H2O, and extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ023 (18 mg, 90%) as a yellow solid. mp 125–127 °C. Rf (hexane/EtOAc = 2/1) = 0.67.
1H-NMR (CDCl3) δ: 1.01 (t, J = 7.44, 3H), 1.43–1.61 (m, 2H), 1.93–2.21 (m, 2H), 5.56 (ddd, J = 5.16, 8.35, 47.6 Hz, 1H), 6.95 (d, J = 2.20 Hz, 1H), 7.27 (dd, J = 1.10, 8.49 Hz, 1H), 7.62 (dd, J = 7.51, 8.49 Hz, 1H), 7.76 (dd, J = 1.10, 7.51 Hz, 1H), 12.14 (s, 1H).
13C-NMR (CDCl3) δ: 13.6 (CH3), 18.0 (d, J = 4.05 Hz, CH2), 35.3 (d, J = 22.4 Hz, CH2), 86.6 (d, J = 171.7 Hz, CH2), 105.9 (d, J = 4.5 Hz, CH), 115.2 (C), 120.1 (CH), 125.4 (CH), 130.7 (d, J = 1.6 Hz, C), 132.6 (C), 136.4 (CH), 152.5 (d, J = 1.5 Hz, C), 159.8 (d, J = 25.7 Hz, C), 162.4 (C), 172.7 (C), 186.2 (C).
19F-NMR (CDCl3): δ −177.5 (s, 1F).
IR (KBr): 756, 1038, 1234, 1316, 1643, 1670.
HRMS (ESI) m/z: [M − H]− Calcd for [C16H12O4F]−, 287.0725. Found, 287.0720.
2-(1-Chloroethyl)-5-hydroxynaphtho[2,3-b]furan-4,9-dione (NQ024)A solution of NQ8019) (50.0 mg, 0.19 mmol) in dichloromethane (10.0 mL) was added dropwise to SOCl2 (70.0 µL, 0.97 mmol) at 0 °C. After 2 h, the mixture was added to pyridine (156 µL, 1.90 mmol) for 0.5 h at r.t., and then concentrated. The residue was purified by silica gel column chromatography gave NQ024 (40 mg, 75%) as a yellow solid. mp 180–182 °C. Rf (hexane/EtOAc = 2/1) = 0.50.
1H-NMR (CDCl3) δ: 1.96 (d, J = 6.99 Hz, 3H), 5.17 (dq, J = 0.56, 6.99 Hz, 1H), 6.92 (d, J = 0.56, 1H), 7.28 (dd, J = 1.17, 8.49 Hz, 1H), 7.63 (dd, J = 7.50, 8.49 Hz, 1H), 7.77 (dd, J = 1.17, 7.50 Hz, 1H), 12.15 (s, 1H)
13C-NMR (CDCl3) δ: 23.0 (CH3), 48.6 (CH), 105.1 (CH), 115.2 (C), 120.1 (CH), 125.4 (CH), 130.8 (C), 132.7 (C), 136.4 (CH), 152.3 (C), 161.7 (C), 162.4 (C), 172.6 (C), 186.2 (C).
IR (KBr): 706, 756, 1034, 1200, 1227, 1246, 1373, 1451, 1539, 1597, 1640, 1667.
HRMS (ESI) m/z: [M + Na]+ Calcd for [C14H9O4ClNa]+, 299.0082. Found, 299.0085.
2-(1-Bromoethyl)-5-hydroxynaphtho[2,3-b]furan-4,9-dione (NQ025)A solution of NQ8019) (50.0 mg, 0.19 mmol) in dichloromethane (10.0 mL) was added dropwise to PBr3 (22.0 µL, 0.23 mmol) at r.t. After 0.5 h, the mixture was concentrated. The residue was purified by silica gel column chromatography gave NQ025 (18 mg, 90%) as a yellow solid. mp 183–185 °C. Rf (hexane/EtOAc = 1/1) = 0.70.
1H-NMR (CDCl3) δ: 2.12 (d, J = 7.03 Hz, 3H), 5.24 (q, J = 7.03 Hz, 1H), 6.92 (s, 1H), 7.28 (dd, J = 1.16, 8.51 Hz, 1H), 7.63 (dd, J = 7.46, 8.51 Hz, 1H), 7.77 (dd, J = 1.16, 7.46 Hz, 1H), 12.15 (s, 1H).
13C-NMR (CDCl3) δ: 23.5 (CH3), 36.4 (CH), 104.8 (CH), 115.2 (C), 120.1 (CH), 125.4 (CH), 130.9 (C), 132.7 (C), 136.4 (CH), 152.1 (C), 162.2 (C), 162.4 (C), 172.6 (C), 186.1 (C).
IR (KBr): 706, 752, 1223, 1242, 1373, 1451, 1535, 1597, 1640, 1662.
HRMS (ESI) m/z: [M + H]+ Calcd for [C14H10O4Br]+, 320.9757. Found, 320.9748.
2-(1-Fluoroethyl)-4,9-dioxo-4,9-dihydronaphtho[2,3-b]furan-5-yl Acetate (NQ031)A solution of NQ008 (38 mg, 0.14 mmol) in DMF (10 mL) was added dropwise to N,N-diisopropylethylamine (DIPEA) (61 µL, 0.35 mmol) and acetyl chloride (20 µL, 0.28 mmol) at 0 °C. After stirred for 1 h, the mixture was extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave 20 (18 mg, 90%) as a yellow solid. mp 138–140 °C. Rf (hexane/EtOAc = 2/1) = 0.67.
1H-NMR (CDCl3) δ: 1.79 (dd, J = 6.78, 23.2 Hz, 3H), 5.71 (dq, J = 1.25, 8.12 Hz, 1H), 6.91 (d, J = 2.52 Hz, 1H), 7.39 (dd, J = 1.25, 8.12 Hz, 1H), 7.77 (dd, J = 7.67, 8.12 Hz, 1H), 8.20 (dd, J = 1.25, 7.67 Hz, 1H), 2.47 (s, 3H)
13C-NMR (CDCl3) δ: 19.2 (d, J = 24.0 Hz, CH3), 21.1 (CH3), 83.1 (d, J = 169.0 Hz, CH), 106.2 (d, J = 4.51 Hz, CH), 124.1 (C), 125.6 (CH), 130.3 (CH), 131.7 (d, J = 1.81 Hz, C), 134.4 (C), 134.9 (CH), 150.4 (C), 151.2 (C), 160.1 (d, J = 24.2 Hz, C), 169.5 (C), 172.5 (C), 179.2(C)
19F-NMR (CDCl3) δ: −169.7 (s, 1F)
IR (KBr): 767.7, 837.1, 1076.3, 1161.2, 1226.7, 1295.4, 1454.3, 1469.8, 1631.8, 1651.1 cm−1
HRMS (ESI) m/z: [M + H]+ Calcd for [C16H12O5F]+, 303.0663. Found, 303.0660.
2-(1-Fluoroethyl)-4,9-dioxo-4,9-dihydronaphtho[2,3-b]furan-5-yl Propionate (NQ032)A solution of NQ008 (50 mg, 0.19 mmol) in DMF (10 mL) was added dropwise to DIPEA (61 µL, 0.35 mmol) and propionyl chloride (34 µL, 0.39 mmol) at 0 °C. After stirred for 1 h, the mixture was extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ032 (46 mg, 75%) as a yellow solid. mp 134–136 °C. Rf (hexane/EtOAc = 2/1) = 0.50.
1H-NMR (CDCl3) δ: 1.34 (t, J = 7.5 Hz, 3H), 1.78 (dd, J = 6.6, 23.3 Hz, 3H), 2.79 (q, J = 7.5 Hz 2H), 5.70 (dq, J = 6.6, 47.7 Hz, 1H), 6.91 (d, J = 2.3 Hz, 1H), 7.38 (dd, J = 1.2, 8.5 Hz 1H), 7.76 (dd, J = 7.5, 8.5 Hz, 1H), 8.19 (dd, J = 1.2, 7.5 Hz, 1H).
13C-NMR (CDCl3) δ: 8.78 (CH3), 19.2 (d, J = 26.4 Hz, CH3), 27.6 (CH2), 83.1 (d, J = 169.8 Hz, CH), 106.2 (d, J = 4.5 Hz, CH), 124.2 (C), 125.5 (CH), 130.3 (CH), 131.7 (d, J = 2.1 Hz, C), 134.3 (CH), 150.5 (C), 151.2 (C), 159.9 (C), 160.2 (C), 172.5 (C), 172.8 (C), 179.2 (C).
19F-NMR (CDCl3) δ: −169.6 (s, 1F).
IR (KBr): 439.7, 702.0, 1142, 1342, 1674, 1759, 2315, 3505.
HRMS (ESI) m/z: [M + Na]+ Calcd for [C17H13O5NaF]+, 339.0645. Found, 339.0648.
2-(1-Fluoroethyl)-4,9-dioxo-4,9-dihydronaphtho[2,3-b]furan-5-yl Heptanoate (NQ033)A solution of NQ008 (50 mg, 0.19 mmol) in DMF (10 mL) was added dropwise to DIPEA (61 µL, 0.35 mmol) and propionyl chloride (52 µL, 0.39 mmol) at 0 °C. After stirred for 1 h, the mixture was extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ033 (35 mg, 51%) as a yellow solid. mp 72–75 °C. Rf (hexane/EtOAc = 2/1) = 0.20.
1H-NMR (CDCl3) δ: 0.95 (t, J = 7.2 Hz, 3H), 1.38–1.48 (m, 4H), 1.78 (dd, J = 6.7, 23.3 Hz, 3H), 1.80–1.86 (m, 2H), 2.75 (t, J = 7.5, 8.1 Hz, 2H), 5.70 (dq, J = 6.6, 47.6 Hz, 1H), 6.91 (d, J = 2.3 Hz 1H), 7.37 (dd, J = 1.2, 7.8 Hz, 1H), 7.76 (dd, J = 7.8, 7.8 Hz, 1H), 8.19 (dd, J = 1.2, 7.8 Hz, 1H).
13C-NMR (CDCl3) δ: 12.3 (CH3), 17.4 (d, J = 24.5 Hz, CH3), 20.7 (CH2), 22.5 (CH2), 29.6 (CH2), 32.5 (CH2), 81.4 (d, J = 168.8 Hz, CH), 104.5 (d, J = 4.6 Hz, CH), 122.6 (C), 123.8 (CH), 128.7 (CH), 130.0 (d, J = 1.4 Hz, C), 132.6 (C), 133.1 (CH), 148.8 (C), 158.2 (C), 158.4 (C), 170.5 (C), 170.9 (C), 177.5 (C).
19F-NMR (CDCl3) δ: −169.6 (s, 1F).
IR (KBr): 471, 872, 1142, 1219, 1589, 1674, 2315, 2955.
HRMS (ESI) m/z: [M + Na]+ Calcd for [C20H19O5NaF]+, 381.1114. Found, 381.1132.
2-(1-Fluoroethyl)-4,9-dioxo-4,9-dihydronaphtho[2,3-b]furan-5-yl 3-methylbutanoate (NQ034)A solution of NQ008 (50 mg, 0.19 mmol) in DMF (10 mL) was added dropwise to DIPEA (61 µL, 0.35 mmol) and isovalenyl chloride (47 µL, 0.39 mmol) at 0 °C. After stirred for 1 h, the mixture was extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ034 (30 mg, 45%) as a yellow solid. mp 73–75 °C. Rf (hexane/EtOAc = 2/1) = 0.50.
1H-NMR (CDCl3) δ: 1.11 (d, J = 6.7 Hz, 6H), 1.78 (dd, J = 6.4, 23.3 Hz, 3H), 2.27–2.35 (m, 1H), 2.65 (d, J = 7.1 Hz, 2H), 5.70 (dq, J = 6.4, 47.8 Hz, 1H), 6.91 (d, J = 2.4 Hz, 1H), 7.36 (dd, J = 1.1, 7.9 Hz, 1H), 7.76 (dd, 7.9, 7.9 Hz, 1H), 8.19 (dd, J = 1.1, 7.9 Hz, 1H).
13C-NMR (CDCl3) δ: 19.2 (d, J = 24.6 Hz, CH3), 22.5 (CH3) 25.3 (CH3), 43.0 (CH2), 83.1 (d, J = 169.6 Hz, CH), 106.2 (d, J = 4.7 Hz, CH), 124.3 (C), 125.5 (CH), 130.4 (CH), 131.7 (d, J = 1.8 Hz, C), 134.3 (C), 134.8 (CH), 150.4 (C), 151.2 (C), 159.9 (C), 160.1 (C), 171.4 (C), 172.5 (C), 179.1 (C).
19F-NMR (CDCl3): δ −169.5 (s, 1F).
IR (KBr): 424, 1026, 1219, 1543, 1674, 2315, 3125.
HRMS (ESI) m/z: [M + Na]+ Calcd for [C19H17O5NaF]+, 367.0958. Found, 367.0971.
2-(1-Fluoroethyl)-4,9-dioxo-4,9-dihydronaphtho[2,3-b]furan-5-yl Benzoate (NQ035)A solution of NQ008 (50 mg, 0.19 mmol) in DMF (10 mL) was added dropwise to DIPEA (61 µL, 0.35 mmol) and benzoyl chloride (54 µL, 0.39 mmol) at 0 °C. After stirred for 1 h, the mixture was extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ035 (59 mg, 84%) as a yellow solid. mp 184–186 °C. Rf (hexane/EtOAc = 4/1) = 0.20.
1H-NMR (CDCl3) δ: 1.75 (dd, J = 6.6, 23.1 Hz, 3H), 5.68 (dq, J = 6.6, 47.9 Hz, 1H), 6.85 (d, J = 2.4 Hz, 1H), 7.52 (dd, J = 1.0, 8.0 Hz, 1H), 7.56 (dd, J = 7.8, 8.1 Hz, 2H), 7.68 (dd, J = 7.4, 8.0 Hz, 1H), 7.81 (dd, J = 7.8, 8.6 Hz, 1H), 8.24 (dd, J = 1.0, 7.4 Hz, 1H), 8.25 (dd, J = 7.8, 8.6 Hz, 2H).
13C-NMR (CDCl3) δ: 19.1 (d, J = 24.3 Hz, CH3), 83.0 (d, J = 168.5 Hz, CH), 106.2 (d, J = 4.3 Hz, CH), 124.3 (C), 125.6 (CH), 128.7 (CH), 129.2 (C), 130.4 (CH), 130.5 (CH), 131.7 (d, J = 1.4 Hz, C), 133.8 (CH), 134.4 (C), 134.9 (CH), 150.6 (2C), 151.2 (C), 159.9 (C), 160.1 (C), 165.1 (C), 172.5 (C), 179.1 (C).
19F-NMR (CDCl3) δ: −169.5 (s, 1F).
IR (KBr): 702, 1088, 1227, 1551, 1674, 2315, 3109.
HRMS (ESI) m/z: [M + Na]+ Calcd for [C21H13O5NaF]+, 387.0645. Found, 387.0636.
2-(1-Hydroxyethyl)-5-((3-methylbut-2-en-1-yl)oxy)naphtho[2,3-b]furan-4,9-dione (NQ036)To a solution of NQ801 (100 mg, 0.39 mmol) and K2CO3 (161 mg, 1.16 mmol) in acetone (10 mL) was added 1-bromo-3-methyl-2-butene (173 µL, 1.16 mmol). After the mixture was refluxed for 1 d, the reaction was quenched by addition of H2O. The mixture was extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ036 (109 mg, 86%) as a yellow solid. mp 128–130 °C. Rf (hexane/EtOAc = 2/1) = 0.40.
1H-NMR (CDCl3) δ: 1.63 (d, J = 6.6 Hz, 3H), 1.77 (s, 3H), 1.80 (s, 3H), 4.73 (d, J = 6.4 Hz, 2H), 5.01 (q, J = 6.6 Hz, 1H), 5.54–5.57 (m, 1H), 6.79 (s, 1H), 7.28 (dd, J = 0.9, 8.5 Hz, 1H), 7.61 (dd, J = 7.8, 8.6 Hz, 1H), 7.80 (dd, J = 0.9, 7.8 Hz, 1H).
13C-NMR (CDCl3) δ: 18.3 (CH3), 21.4 (CH3), 25.8 (CH3), 63.7 (CH2), 66.6 (CH), 104.1 (CH), 119.0 (CH), 119.7 (CH), 120.3 (CH), 120.5 (C), 132.9 (C), 134.7 (CH), 135.0 (C), 138.4 (C), 150.1 (C), 159.8 (C), 165.6 (C), 173.0 (C), 180.1 (C).
IR (KBr): 718, 925, 1026, 1219, 1589, 1667, 2315, 3321.
HRMS (ESI) m/z: [M + Na]+ Calcd for [C19H18O5Na]+, 349.1052. Found, 349.1086.
2-(1-Fluoroethyl)-5-((3-methylbut-2-en-1-yl)oxy)naphtho[2,3-b]furan-4,9-dione (NQ038)To a solution of NQ036 (100 mg, 0.30 mmol) in dichloromethane (DCM) (10 mL) was added (Diethylamino)sulfur Trifluoride (DAST) (65 µL, 0.45 mmol) at 0 °C. After stirred for 1 d at r.t., the reaction was quenched by addition of H2O. The mixture was extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ038 (82 mg, 81%) as a yellow solid. mp 128–130 °C. Rf (hexane/EtOAc = 2/1) = 0.70.
1H-NMR (CDCl3) δ: 1.75–1.82 (m, 9H), 4.74 (d, J = 6.0 Hz, 2H), 5.55–5.59 (m, 1H), 5.70 (ddd, J = 6.3, 12.9, 47.8 Hz, 1H), 6.29 (d, J = 2.6 Hz, 1H), 7.32 (dd, J = 0.9, 8.5 Hz, 1H), 7.65 (dd, J = 7.6, 8.6 Hz, 1H), 7.80 (dd, J = 0.9, 7.6 Hz, 1H).
13C-NMR (CDCl3) δ: 18.4 (CH3), 19.1 (d, J = 24.1 Hz, CH3), 25.8 (CH3), 66.6 (CH2), 83.2 (d, J = 168.5 Hz, CH), 106.5 (d, J = 4.8 Hz, CH), 119.1 (CH), 119.8 (CH), 120.4 (CH), 120.6 (C), 134.2 (d, J = 1.7 Hz, C), 134.7 (CH), 135.0 (C), 138.6 (C), 159.5 (C), 157.5 (C), 159.9 (C), 173.3 (C), 179.5 (C).
19F-NMR (CDCl3) δ: −168.8 (s, 1F).
IR (KBr): 702, 1219, 1528, 1674, 2315, 2963, 3433.
HRMS (ESI) m/z: [M + Na]+ Calcd for [C19H17FO4Na]+, 351.1009. Found, 351.1027.
5-(Benzyloxy)-2-(1-hydroxyethyl)naphtho[2,3-b]furan-4,9-dione (NQ037)To a solution of NQ801 (100 mg, 0.39 mmol) and K2CO3 (161 mg, 1.16 mmol) in acetone (10 mL) was added benzyl bromide (138 µL, 1.16 mmol). After the mixture was refluxed for 1 d, the reaction was quenched by addition of H2O. The mixture was extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ037 (81 mg, 60%) as a yellow solid. mp 164–167 °C. Rf (hexane/EtOAc = 2/1) = 0.20.
1H-NMR (CDCl3) δ: 1.63 (d, J = 6.6 Hz, 3H), 2.58 (s, 1H), 5.00 (q, J = 6.5 Hz, 1H), 5.29 (s, 1H), 6.81 (s, 1H), 7.31–7.34 (m, 2H), 7.42 (dd, J = 7.7, 8.1 Hz, 2H), 7.58–7.63 (m, 3H), 7.86 (dd, J = 0.9, 7.5 Hz, 1H).
13C-NMR (CDCl3) δ: 21.4 (CH3), 63.8 (CH), 71.0 (CH2), 104.2 (CH), 120.1 (CH), 120.2 (CH), 120.8 (C), 126.7 (CH), 128.0 (CH), 128.7 (CH), 132.8 (C), 134.8 (CH), 135.0 (C), 136.1 (C), 150.3 (C), 159.4 (C), 165.4 (C), 173.1 (C), 180.1 (C).
IR (KBr): 702, 1142, 1342, 1674, 1759, 2315, 3505.
HRMS (ESI) m/z: [M + Na]+ Calcd for [C21H16O5Na]+, 371.0895. Found, 371.0887.
5-(Benzyloxy)-2-(1-fluoroethyl)naphtho[2,3-b]furan-4,9-dione (NQ039)To a solution of NQ037 (100 mg, 0.30 mmol) in DCM (10 mL) was added DAST (65 µL, 0.45 mmol) at 0 °C. After stirred for 1 d at r.t., the reaction was quenched by addition of H2O. The mixture was extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4, and then concentrated. The residue was purified by silica gel column chromatography gave NQ039 (90 mg, 89%) as a yellow solid. mp 162–165 °C. Rf (hexane/EtOAc = 2/1) = 0.60.
1H-NMR (CDCl3) δ: 1.77 (dd, J = 6.6, 23.1 Hz, 3H), 5.27 (s, 2H), 5.68 (ddd, J = 6.6, 10.0 Hz. 47.8 Hz, 1H), 6.93 (d, J = 2.4 Hz, 1H), 7.31–7.35 (m, 2H), 7.41 (dd, J = 7.6, 8.0 Hz, 2H), 7.58–7.63 (m, 3H), 7.86 (dd, J = 0.8, 7.6 Hz, 1H).
13C-NMR (CDCl3) δ: 19.1 (d, J = 23.5 Hz, CH3), 71.0 (CH2), 83.1 (d, J = 169.5 Hz, CH), 106.5 (d, J = 4.7 Hz, CH), 120.2 (d, J = 21.7 Hz, CH), 120.7 (C), 126.7 (CH), 127.9 (CH), 128.7 (CH), 132.4 (d, J = 1.9 Hz, C), 134.8 (CH), 135.0 (C), 136.0 (C), 150.7 (d, J = 1.7 Hz, C), 159.4 (C), 159.6 (C), 159.8 (C), 173.1 (C), 179.5 (C).
19F-NMR (CDCl3) δ: −168.8 (s, 1F).
IR (KBr): 764, 1034, 1234, 1528, 1621, 2315, 3480.
HRMS (ESI) m/z: [M + Na]+ Calcd for [C21H15FO4Na]+, 373.0852. Found, 373.0862.
The authors are grateful to Taheebo Japan Co., Ltd. and the late Tetsuro Fujita, professor emeritus of Kyoto University, for their generous financial support to this project. The authors thank Kazunori Ueda for the contribution of the synthesis of the compounds.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.