2019 年 67 巻 7 号 p. 675-689
2019 年 67 巻 7 号 p. 675-689
An Orobanchaceae plant Cistanche tubulosa (SCHENK) WIGHT (Kanka-nikujuyou in Japanese), which is one of the authorized plant resources as Cistanches Herba in both Japanese and Chinese Pharmacopoeias, is a perennial parasitic plant growing on roots of sand-fixing plants. The stems of C. tubulosa have traditionally been used for treatment of impotence, sterility, lumbago, and body weakness as well as a promoting agent of blood circulation. In recent years, Cistanches Herba has also been widely used as a health food supplement in Japan, China, and Southeast Asian countries. Here we review our recent studies on chemical constituents from the stems of C. tubulosa as well as their bioactivities such as vasorelaxtant, hepatoprotective, and glucose tolerance improving effects.
The genus Cistanche comprises parasitic plants belonging to the Orobanchaceae family. They commonly attach onto the roots of sand-fixing plants, such as Haloxylon ammodendron, Haloxylon persicum, Kalidium foliatum, and Tamarix plants, etc. The Cistanche species are distributed mainly in arid lands and deserts across Eurasia and North Africa, such as China, Iran, India, and Mongolia.1–6) Among them, China has experienced severe land desertification especially in the northwest regions, in particular Inner Mongolia and Xinjiang autonomous regions.7,8) Construction of shelter forests is regarded as one of the best ways to turn deserts into the oases, so that the common hosts of Cistanche plants are widely believed as suitable sand-fixing candidates. The combination of parasitic Cistanche plants with their hosts could be not only a bright prospect for the development of oases, but also for remarkable economic outcomes, because Cistanches Herba (Rou Cong-Rong in Chinese), the dried succulent stems of Cistanche plants including Cistanche deserticola Y. C. MA, Cistanche tubulosa (SCHENK) WIGHT, Cistanche salsa (C. A. MEY) G. BECK, and Cistanche sinensis BECK, are one of the most famous edible and medicinal plants.7–10) Among them, the dried stems of C. salsa, C. deserticola, and C. tubulosa are listed as a crude drug “Nikujuyou” in the Japanese Pharmacopoeia.7) As for the Chinese Pharmacopoeia, the dried stems of C. deserticola and C. tubulosa are authorized,8) and other non-official species, C. salsa and C. sinensis, are also used in certain regions of China due to resource shortage.4) Cistanches Herba comprises one of the most valuable plants in traditional Chinese medicine (TCM), which supplements kidney functions, boosts the essence of blood, and moistens the large intestines to free stool.4) Among all the tonics in TCM, it is widely accepted as a superior one and has even been given the name “ginseng of the desert” or “desert ginseng.”2–4) Furthermore, it is frequently prescribed to treat chronic renal disease, impotence, female infertility, morbid leucorrhea, profuse metrorrhagia, and senile constipation in TCM.3) Cistanches Herba has reported pharmacological activities expected for the treatment of neurodegenerative disorders11–14) such as Alzheimer’s disease,15) Parkinson’s disease,16–18) and vascular dementia.19) Moreover, Cistanche Herba is widely used as a health food supplement in Japan, China, and southeast Asian countries.20) In the course of our characterization studies on bioactive constituents from medicinal herbs originating in Xinjiang autonomous region and neighboring areas in China,21–30) we have reported an exhaustive study on the stems of C. tubulosa.21–26) Here we review our studies on chemical constituents from the stems of C. tubulosa as well as their biological activities.
The stems of C. tubulosa (Kanka-nikujuyou in Japanese; Fig. 1), which is one of the authorized plants as Cistanches Herba in both Japanese and Chinese Pharmacopoeias,7,8) have been used traditionally for treating impotence, sterility, lumbago, and body weakness.1) Previously, several therapeutic effects of the extract from the stems of C. tubulosa were reported in experimental animal models such as anti-hyperglycemic,31) hypolipidemic,31) hypocholesterolemic,32) and anti-inflammatory effects,33) and amelioration of dextran sulphate sodium (DSS)-induced colitis.34) Moreover, several iridoids, monoterpenoids, phenylethanoids, and lignens were isolated from Chinese and Pakistani C. tubulosa.35–39) During the course our chemical studies on the stems of C. tubulosa, we previously reported the isolation and structure determination of varieties monoterpenoids including iridoids, phenylethanoid glycosides and related sugar esters, and other isolates such as phenylpropanoid glycosides, lignin glycosides, alkyl glycosides, and sugar alcohol.21–25)

Iridoids are a type of monoterpenoid constructed from 10-carbon skeleton of isoprene building units having a cyclopentanopyran ring system. The subclass, secoidiroids, are cleaved form in the cyclopropane or pyran ring. Iridoids and secoisidoids are often found in medicinal plants as glycosides, mainly glucosides. These medicinal plants have been traditionally used as bitter tonics, sedatives, diuretics, cough medicines, remedies for wounds, nervous and skin disorders, obesity, epilepsy, insanity, snake poisoning, diabetic, and hyperlipidemic disorders. A number of reviews on naturally occurring iridoids and secoiridoids have covered aspects of the chemical diversity40–46) and varieties of the biological and pharmacological activities e.g. antibacterial, antifungal, anticancer, antidiabetic, antihyperlipidemic, anti-osteoporosis, antioxidant, anti-protozoal, hepatoprotective, immunomodulatory, neuroprotective, and neuritogenic activities.44–50)
In our structure identification and elucidation studies of iridoid constituents from the stems of C. tubulosa, seven new and nine known iridoid glycosides such as kankanosides A21) (1), B21) (2), C21) (3), D21) (4), L25) (5), M25) (6), and N25) (7), mussaenosidic acid51) (8), geniposidic acid51) (9), 8-epiloganic acid39) (10), 8-epideoxyloganic acid51) (11), gluroside51) (12), antirrhide52) (13), ajugol51) (14), bartsioside51) (15), and 6-deoxycatalpol51) (16) and a new and three known iridoids, kankanol21) (17), argyol53) (18), cistanin54) (19), and cistanchlorin54) (20) were obtained (Chart 1). As for aliphatic monoterpene glycosides, three new and four known compounds such as kankanosides E21) (21), O25) (22), and P25) (23), (2E,6Z)-8-β-D-glucopyranosyloxy-2,6-dimethyl-2,6-octadienoic acid55) (24), (2E,6E)-3,7-dimethyl-8-hydroxyoctadien-1-yl-O-β-D-glucopyranoside56) (25), 8-hydroxygeraniol 8-O-β-D-glucopyranoside57) (26), and betulalbuside A58) (27) were also obtained from the stems of C. tubulosa (Chart 2). Among them, the noriridoid glycosides, gluroside (12), bartsioside (15), and 6-deoxycatalpol (16), were isolated as the principal constituents from this plant material.21,25)


Phenylethanoid glycosides are a type of phenolic compound characterized by a β-glucopyranoside structure bearing a hydroxyphenylethyl moiety as the aglycone. These compounds often comprise a number of acyl groups such as cinnamic acid, p-coumaric acid, caffeic acid, ferulic acid, isoferulic acid, etc. and/or various sugars (e.g., rhamnose, xylose, apiose, arabinose, etc.) attached to the glucose residue through ester or glycosidic linkages, respectively. They mainly have been found in Scrophulariaceae, Oleaceae, Plantaginaceae, Lamiaceae, and Orobanchaceae families.59–61) Among them, echinacoside62,63) (28) and acteoside64–67) (29, also called verbascoside, kusaginin, and orobanchin) are representatives of the well-studied phenylethanoid glycosides and have been reported to possess a number of important bioactive properties such as antioxidative, neuroprotective, nitric oxide radical scavenging, antihepatotoxic, and antiosteoporotic activities.2–4,6,59–61,68–77)
As phenylethanoid glycoside constituents from the stems of C. tubulosa, we have isolated the above-mentioned echinacoside78) (28) and acteoside78) (29) as the major compounds along with nine new and 17 known compounds such as kankanosides F22) (30), G22) (31), H123) (32), H223) (33), I23) (34), J124) (35), J224) (36), K124) (37), and K224) (38), isoacteoside78) (39), cis-acteoside79,80) (40), 2′-acetylacteoside78) (41), decaffeoylacteoside81) (42), tubuloside A78) (43), cistantubulosides B182) (44) and B282) (45), arenarioside83) (46), wiedemanninoside C84) (47), tubuloside B78) (48), syringalide A 3′-O-α-L-rhamnopyranoside85) (49), cistantubuloside A82) (50), cistanoside G81) (51), salidroside81) (52), campneoside I86,87) (53), isocampneoside I88) (54), and campneoside II86,87) (55). Furthermore, a new and a known corresponding sugar ester, kankanose22) (56) and cistanoside F89) (57), were also obtained (Chart 3).

As shown in Chart 4, the other isolates have been obtained such as: (i) phenylpropanoids: coniferin90) (58), syringin90) (59), and sinapic aldehyde 4-O-β-D-glucopyranoside91) (60), (ii) lignans: (+)-pinoresinol O-β-D-glucopyranoside92) (61), eucommin A93) (62), isoeucommin94) (63), and (+)-syringaresinol O-β-D-glucopyranoside95) (64), (iii) alkyl glycosides: (2R,3R)-butane-2,3-diol 2-O-β-D-glucopyranoside96) (65), meso-butane-2,3-diol 2-O-β-D-glucopyranoside96) (66), ethyl β-D-glucopyranoside96) (67), and 3-methylbutan-1-ol β-D-glucopyranoside96) (68), and (iv) sugar alcohol: D-mannitol (69). Among the isolates, D-mannitol (69) gave the most highest isolation yield in our study.

It is well known that high concentration of potassium cation (high K+)-induced contractions in smooth muscles are the result of an increase in intracellular calcium ion (Ca2+), and calcium channel blocker such as nifedipine inhibit the voltage-dependent calcium channel, thereby inhibiting contractions in depolarized aortic strips, but they show weak inhibitory effects on noradrenaline (NA)-induced contractions.97) We have reported that several sesquiterpenoids and diarylheptanoids isolated from Zedoariae Rhizoma (Zingiberaceae) showed inhibitory effects on contractions induced by high K+ in isolated rat aortic strips, while they did not inhibit NA-induced contractions.98,99) In contrast, the principal phenylethanoid glycosides in the stems of C. tubulosa, echinacoside (28) and acteoside (29), and the related isolates, kankanoside F (30), isoacteoside (39), kankanose (56), and cistanoside F (57), were found to show vasorelaxant activity induced by NA contractions (Table 1), whereas they did not inhibit high K+-induced contractions22) (Table 2). These findings suggested that these active constituents inhibited contractions via receptor-operated calcium channel, but not via voltage-dependent calcium channel. Especially, echinacoside (28) and acteoside (29) having the 4′-O-caffeoyl group in the 8-O-β-D-glucopyranosyl part significantly inhibited NA-induced contractions, time- and concentration-dependently, while isoacteoside (39) having the 6′-O-caffeoyl group showed weaker activity [echinacoside (28), acteoside (29) > isoacteoside (39)]. Furthermore, the relaxant effects of echinacoside (28) and acteoside (29) were observed approx. 30 min after addition of NA in a different way from that of prazosin, an adrenaline α1-receptor antagonist (Fig. 2). The mechanisms of action of this behavior should be studied further.
| Conc. | Time | Contraction (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 5 min | 10 min | 20 min | 30 min | 40 min | 50 min | 60 min | ||
| Control | — | 99.5 ± 0.5 | 100.4 ± 0.8 | 100.1 ± 0.7 | 100.0 ± 0.3 | 100.2 ± 0.2 | 99.4 ± 0.4 | 99.7 ± 0.7 |
| Methanol extract | 30 µg/mL | 99.3 ± 0.6 | 99.3 ± 0.9 | 99.3 ± 1.7 | 97.7 ± 1.9 | 95.4 ± 2.1 | 90.3 ± 3.7 | 78.8 ± 9.0b) |
| 100 µg/mL | 100.1 ± 0.5 | 100.0 ± 0.9 | 98.1 ± 1.7 | 89.6 ± 7.4 | 75.2 ± 15.3 | 52.3 ± 14.4b) | 19.3 ± 9.2b) | |
| 300 µg/mL | 101.8 ± 0.7 | 99.8 ± 0.8 | 88.4 ± 5.2 | 55.9 ± 13.6b) | 23.0 ± 11.0b) | 6.7 ± 4.1b) | 1.9 ± 1.2b) | |
| Control | — | 99.7 ± 0.2 | 99.6 ± 0.4 | 100.3 ± 1.0 | 100.5 ± 1.5 | 100.4 ± 1.4 | 100.9 ± 1.8 | 100.6 ± 1.9 |
| Echinacoside (28) | 10 µM | 100.0 ± 0.0 | 99.6 ± 0.6 | 92.6 ± 2.5 | 74.0 ± 7.9 | 32.0 ± 6.7b) | 5.5 ± 1.3b) | 0.4 ± 0.4b) |
| 30 µM | 99.5 ± 0.7 | 99.9 ± 1.7 | 88.5 ± 6.7 | 56.7 ± 16.3 | 24.5 ± 11.7b) | 7.4 ± 3.9b) | 3.0 ± 2.1b) | |
| 100 µM | 100.0 ± 0.0 | 99.1 ± 0.9 | 82.4 ± 7.8 | 35.4 ± 17.5b) | 14.9 ± 12.9b) | 5.9 ± 5.9b) | 2.8 ± 2.8b) | |
| Acteoside (29) | 10 µM | 103.1 ± 3.9 | 102.2 ± 5.7 | 91.6 ± 10.9 | 67.8 ± 21.0 | 55.1 ± 21.3a) | 41.5 ± 20.2b) | 29.6 ± 16.4b) |
| 30 µM | 96.0 ± 1.8 | 91.9 ± 3.1 | 73.2 ± 8.6a) | 45.6 ± 13.4a) | 20.3 ± 9.4b) | 5.4 ± 4.0b) | 1.6 ± 1.6b) | |
| 100 µM | 96.2 ± 1.9 | 91.6 ± 4.5 | 83.0 ± 9.7 | 53.3 ± 16.0a) | 23.3 ± 11.4b) | 8.1 ± 5.2b) | 2.8 ± 2.8b) | |
| Isoacteoside (39) | 10 µM | 100.6 ± 0.3 | 101.1 ± 0.4 | 101.3 ± 0.4 | 100.7 ± 0.9 | 98.8 ± 1.6 | 96.4 ± 2.3 | 89.1 ± 6.2 |
| 30 µM | 99.6 ± 0.3 | 99.5 ± 0.5 | 98.5 ± 0.4 | 96.1 ± 0.5 | 90.9 ± 1.9 | 87.5 ± 5.1 | 72.0 ± 8.2 | |
| 100 µM | 99.9 ± 1.0 | 101.1 ± 0.7 | 100.4 ± 1.0 | 97.6 ± 1.8 | 90.6 ± 3.8 | 76.9 ± 6.8 | 59.6 ± 9.9b) | |
| Control | — | 101.1 ± 0.1 | 99.9 ± 0.5 | 100.3 ± 0.3 | 100.6 ± 0.3 | 100.9 ± 0.3 | 100.1 ± 0.7 | 100.5 ± 0.9 |
| Kankanoside F (30) | 100 µM | 98.8 ± 0.4 | 97.1 ± 1.7 | 31.3 ± 14.7b) | 2.5 ± 1.8b) | 0.0 ± 0.0b) | 0.0 ± 0.0b) | 0.0 ± 0.0b) |
| Salidroside (52) | 100 µM | 99.7 ± 0.2 | 99.9 ± 0.3 | 99.9 ± 0.3 | 98.6 ± 0.6 | 98.3 ± 0.8 | 97.4 ± 1.3 | 96.3 ± 1.9 |
| Kankanose (56) | 100 µM | 97.7 ± 0.7 | 96.2 ± 2.7 | 65.8 ± 14.4 | 9.6 ± 4.4b) | 0.0 ± 0.0b) | 0.0 ± 0.0b) | 0.0 ± 0.0b) |
| Cistanoside F (57) | 100 µM | 98.8 ± 0.5 | 97.0 ± 1.3 | 30.1 ± 12.4b) | 3.5 ± 1.3b) | 0.4 ± 0.4b) | 0.0 ± 0.0b) | 0.0 ± 0.0b) |
| Caffeic acid | 10 µM | 102.1 ± 1.7 | 103.6 ± 2.2 | 103.3 ± 3.2 | 91.7 ± 11.7 | 73.9 ± 22.8 | 57.3 ± 23.8b) | 43.9 ± 22.5b) |
| 30 µM | 103.6 ± 2.0 | 105.8 ± 3.3 | 95.4 ± 3.2 | 43.6 ± 14.2a) | 9.9 ± 7.4b) | 0.9 ± 0.9b) | 0.0 ± 0.0b) | |
| 100 µM | 99.2 ± 0.6 | 88.2 ± 9.0 | 73.3 ± 10.7b) | 30.6 ± 8.3b) | 3.0 ± 0.6b) | 0.0 ± 0.0b) | 0.0 ± 0.0b) | |
| Prazosin | 10 nM | 83.0 ± 6.8b) | 64.4 ± 9.3b) | 33.6 ± 4.8b) | 27.7 ± 3.8b) | 25.0 ± 3.7b) | 24.4 ± 3.0b) | 22.6 ± 3.0b) |
| 100 nM | 7.2 ± 0.3b) | 0.3 ± 0.2b) | 0.0 ± 0.0b) | 0.0 ± 0.0b) | 0.0 ± 0.0b) | 0.0 ± 0.0b) | 0.0 ± 0.0b) | |
Each value represents the mean ± standard error of the mean (S.E.M.) (n = 4–8). Significantly different from control: a) p < 0.05; b) p < 0.01. Reproduced with permission from Bioorg. Med. Chem., 14, 7468–7475. Copyright [2006]. Elsevier.
| Conc. | Time | Contraction (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 5 min | 10 min | 20 min | 30 min | 40 min | 50 min | 60 min | ||
| Control | — | 99.7 ± 0.3 | 99.7 ± 0.8 | 102.0 ± 0.9 | 102.5 ± 1.2 | 102.3 ± 1.0 | 102.3 ± 1.0 | 102.1 ± 2.1 |
| Echinacoside (28) | 100 µM | 101.0 ± 0.2 | 100.4 ± 0.4 | 101.7 ± 0.9 | 100.4 ± 0.4 | 100.2 ± 0.2 | 101.3 ± 1.3 | 100.9 ± 1.7 |
| Acteoside (29) | 100 µM | 101.0 ± 0.5 | 103.4 ± 0.9 | 104.0 ± 0.7 | 105.3 ± 0.4 | 105.1 ± 0.4 | 105.2 ± 0.3 | 105.1 ± 0.4 |
| Kankanoside F (30) | 100 µM | 102.4 ± 0.3 | 102.6 ± 0.8 | 103.2 ± 0.4 | 103.2 ± 0.4 | 104.2 ± 2.2 | 103.9 ± 2.5 | 104.8 ± 3.4 |
| Isoacteoside (39) | 100 µM | 102.7 ± 1.5 | 104.1 ± 1.9 | 105.4 ± 2.0 | 105.7 ± 1.8 | 105.5 ± 0.9 | 105.2 ± 0.8 | 105.7 ± 1.0 |
| Salidroside (52) | 100 µM | 100.3 ± 0.3 | 100.0 ± 0.6 | 100.8 ± 0.8 | 100.0 ± 0.0 | 99.7 ± 0.3 | 100.0 ± 0.6 | 99.5 ± 0.5 |
| Kankanose (56) | 100 µM | 100.9 ± 0.5 | 102.1 ± 1.3 | 103.3 ± 1.4 | 103.6 ± 1.7 | 103.6 ± 1.7 | 103.2 ± 2.1 | 103.1 ± 2.3 |
| Cistanoside F (57) | 100 µM | 100.6 ± 0.6 | 101.2 ± 1.2 | 102.3 ± 1.8 | 103.2 ± 2.7 | 103.0 ± 3.0 | 103.3 ± 3.3 | 103.3 ± 3.3 |
| Caffeic acid | 100 µM | 101.6 ± 0.0 | 103.4 ± 1.0 | 103.9 ± 1.6 | 103.8 ± 2.2 | 103.1 ± 2.4 | 103.0 ± 1.4 | 103.3 ± 1.4 |
| Control | — | 100.1 ± 0.3 | 101.0 ± 0.6 | 102.1 ± 0.7 | 102.3 ± 0.9 | 102.7 ± 1.0 | 102.5 ± 0.9 | 102.7 ± 1.0 |
| Nifedipine | 1 nM | 98.6 ± 0.3 | 96.6 ± 0.4 | 94.4 ± 0.6b) | 93.1 ± 1.4b) | 91.5 ± 2.0b) | 90.3 ± 2.2b) | 89.9 ± 2.4b) |
| 10 nM | 85.6 ± 1.1b) | 72.9 ± 1.2b) | 58.4 ± 1.5b) | 52.5 ± 1.4b) | 49.5 ± 1.6b) | 48.8 ± 1.7b) | 48.5 ± 1.7b) | |
| 100 nM | 55.6 ± 2.6b) | 29.6 ± 2.0b) | 16.2 ± 1.9b) | 13.9 ± 1.8b) | 13.6 ± 1.9b) | 13.4 ± 1.9b) | 13.4 ± 2.0b) | |
Each value represents the mean ± S.E.M. (n = 4–6). Significantly different from control: a) p < 0.05; b) p < 0.01.

Reproduced with permission from Bioorg. Med. Chem., 14, 7468–7475. Copyright [2006]. Elsevier.
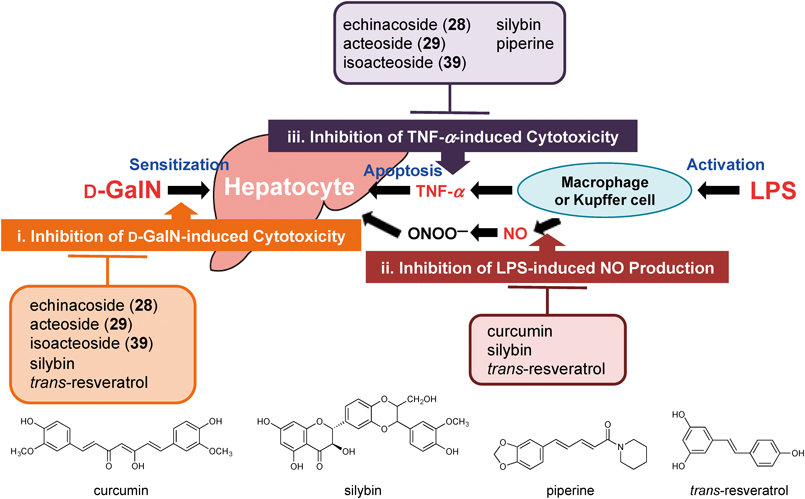
Infection with hepatitis C virus and chronic consumption of alcohol are major causes of liver injury, cirrhosis, and hepatocellular carcinoma worldwide. Tumor necrosis factor-α (TNF-α) is known to mediate organ injuries through its induction of cellular inflammatory responses. In the liver, the biological effects of TNF-α have been implicated in hepatic injuries associated with hepatic toxins, ischemia/reperfusion, viral hepatitis, and alcoholic liver disease or alcohol-related disorders.100–102) Therefore TNF-α is considered as an important target in the attempt to discover anti-inflammatory and hepatoprotective agents. The D-galactosamine (D-GalN)/lipopolysaccharide (LPS)-induced liver injury model is recognized to develop via immunological responses.103) This model causes liver injury in two steps. First, expression of inhibitors of apoptosis proteins (IAPs) is inhibited by administration of D-GalN through depletion of uridine triphosphate in hepatocytes. Second, proinflammatory mediators such as nitric oxide (NO), reactive oxygen species (ROS), and TNF-α are released from LPS-activated macrophages (Kupffer’s cells). Apoptosis of hepatocytes induced by TNF-α plays an important role in D-GalN/LPS-induced liver injury.104) In our previous studies on hepatoprotective properties of compounds obtained from natural resources, we reported that sesquiterpenoids,105–108) diarylheptanoids,105–108) saponins,109) coumarins,110) acid amides,111–113) triterpenoids,114) limonoids,115) and stilbenoids116) exhibited significant protective effects against liver injuries induced by D-GalN/LPS in mice. The methanol extract from the stems of C. tubulosa and its principal phenylethanolid glycosides, echinacoside (28), acteoside (29), and isoacteoside (39), showed inhibitory effects on the increases in serum aspartate aminotransaminase (sAST) and alanine aminotransaminase (sALT), markers of liver injury, induced by D-GalN/LPS in mice23) (Table 3). Notably, these isolates (28, 29, and 39) were equivalent to curcumin117–119) obtained from turmeric (Curcuma longa) and more potent than silybin120–124) obtained from milk thistle (Silybum marianum), both of which are well-recognized naturally occurring hepatoprotective products.
| Treatment | Dose (mg/kg, p.o.) | n | sAST | sALT | ||
|---|---|---|---|---|---|---|
| (Karmen unit) | Inhibition (%) | (Karmen unit) | Inhibition (%) | |||
| Normal (vehicle) | — | 7 | 86 ± 5b) | — | 28 ± 6b) | — |
| Control (D-GalN/LPS) | — | 11 | 10714 ± 1520 | — | 6823 ± 1011 | — |
| Methanol extract | 250 | 8 | 4653 ± 1698b) | 56.6 | 3632 ± 1527 | 46.8 |
| 500 | 8 | 2049 ± 556b) | 80.9 | 1318 ± 397b) | 80.7 | |
| 1000 | 8 | 904 ± 272b) | 91.6 | 701 ± 226b) | 89.7 | |
| Normal (vehicle) | — | 5 | 58 ± 6b) | — | 25 ± 2b) | — |
| Control (D-GalN/LPS) | — | 12 | 11768 ± 1621 | — | 5484 ± 666 | — |
| Echinacoside (28) | 25 | 8 | 4562 ± 1413a) | 61.2 | 3084 ± 1117 | 43.8 |
| 100 | 8 | 3914 ± 1181b) | 66.7 | 2634 ± 920 | 52.0 | |
| Acteoside (29) | 25 | 8 | 5736 ± 3048a) | 51.3 | 3047 ± 1462 | 44.4 |
| 100 | 8 | 3703 ± 1594b) | 68.5 | 2220 ± 1045a) | 59.5 | |
| Isoacteoside (39) | 25 | 8 | 6339 ± 1950 | 46.1 | 3278 ± 1021 | 40.2 |
| 100 | 8 | 3425 ± 848b) | 70.9 | 2265 ± 567a) | 58.7 | |
| Normal (vehicle) | — | 10 | 55 ± 5b) | — | 17 ± 1b) | — |
| Control (D-GalN/LPS) | — | 10 | 6033 ± 1647 | — | 6605 ± 1985 | — |
| Curcumin105–108,114–116) | 12.5 | 10 | 4770 ± 1218 | 21.1 | 5024 ± 1189 | 24.0 |
| 25 | 10 | 3177 ± 979 | 47.8 | 3253 ± 981 | 50.9 | |
| 50 | 9 | 2220 ± 563a) | 63.8 | 1916 ± 483a) | 71.2 | |
| Control (D-GalN/LPS) | — | 10 | 4709 ± 461 | — | 7088 ± 917 | — |
| Silybinc),115,116) | 500 | 8 | 1361 ± 191b) | 71.1 | 1990 ± 439b) | 71.9 |
| Normal (vehicle) | — | 5 | 95 ± 5b) | — | 19 ± 1b) | — |
| Control (D-GalN/LPS) | — | 8 | 9126 ± 1477 | — | 9830 ± 1605 | — |
| Hydrocortisone | 10 | 7 | 627 ± 262b) | 94.2 | 247 ± 123b) | 97.7 |
Each value represents the mean ± S.E.M. Significantly different from control: a) p < 0.05; b) p < 0.01. c) Commercial silybin was purchased from Funakoshi Co., Ltd. (Tokyo, Japan). Reproduced with permission from Bioorg. Med. Chem., 18, 1882–1890. Copyright [2010]. Elsevier.
To characterize the mechanisms responsible for the hepatoprotective activity, the inhibitory effects of the isolates on D-GalN-induced cytotoxicity in primary cultured mouse hepatocytes were examined. Using this in vitro assay, we previously reported several active constituents from the following natural resources such as Curcuma zedoaria,105–108) Anastatica hierochuntica,125) Cyperus longus,126) Erycibe expansa,127) Angelica furcijuga,110,128) Camellia sinensis,129) Sedum sarmentosum,130,131) Rhodiola sachalinensis,132) Sinocrassula indica,133) Hedychium coronarium,134) Piper chaba,111–113) Potentilla anserina,114) Cassia auriculata,135) and Shorea roxburghii.116) As for the isolates of C. tubulosa, several phenylethanoid glycosides, echinacoside (28, IC50 = 10.2 µM), acteoside (29, 4.6 µM), kankanoside G (31, 14.8 µM), isoacteoside (39, 5.3 µM), 2′-acetylacteoside (41, 4.8 µM), tubulosides A (43, 8.6 µM) and B (48, 14.6 µM), and syringalide A 3′-O-α-L-rhamnopyranoside (49, 71.2 µM), and lignans, (+)-pinoresinol O-β-D-glucopyranoside (61, 48.5 µM) and (+)-syringaresinol O-β-D-glucopyranoside (64, 98.4 µM) showed inhibitory activity, whereas none of the monoterpenoids including iridoid constituents led to a reduction in cytotoxicity at concentrations of up to 100 µM23) (Table 4). The hepatoprotective activities of phenylethanoid glycosides (28, 29, 31, 39, 41, 43, and 48) were greater than that of commercial silybin (IC50 = 38.8 µM).115,116) Furthermore, the structural requirements of the phenylethanoid glycosides for hepatoprotective activity were: (i) the aglycone part was essential for activity [echinacoside (28) >> kankanose (56, > 100 µM); acteoside (29) >> cistanoside F (57, > 100 µM)]; (ii) the aglycone having the 3,4-dihydroxy group showed stronger activity than that having the 4-hydroxy group [isoacteoside (39) > kankanoside G (31)]; (iii) the 6′-O-β-D-glucopyranosyl moiety reduced the activity [acteoside (29) > echinacoside (28); 2′-acetylacteoside (41) > tubuloside A (43)]; (iv) the 8-O-β-D-glucopyranosyl part having the 4′-O-caffeoyl group showed stronger activity than that having the 6′-O-caffeoryl group [acteoside (29) ≧ isoacteoside (39); 2′-acetylacteoside (41) > tubuloside B (48)]; and (v) introduction of the 2′-O-acetyl moiety reduced the activity [acteoside (29) ≧ 2′-acetylacteoside (41); isoacteoside (39) > tubuloside B (48)].
| Inhibition (%) | IC50 (µg/mL) | |||||
|---|---|---|---|---|---|---|
| 0 µg/mL | 3 µg/mL | 10 µg/mL | 30 µg/mL | 100 µg/mL | ||
| Methanol extract | 0.0 ± 1.8 | 9.1 ± 2.9a) | 17.3 ± 1.9b) | 29.2 ± 1.4b) | 53.0 ± 2.4b) | 97.3 |
| Inhibition (%) | IC50 (µM) | |||||
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | ||
| Kankanoside B (2) | 0.0 ± 0.9 | −0.2 ± 1.8 | −2.5 ± 0.7 | −1.0 ± 2.2 | 0.6 ± 1.5 | |
| Kankanoside C (3) | 0.0 ± 3.1 | −3.0 ± 3.6 | −1.7 ± 4.6 | −3.9 ± 1.9 | 7.1 ± 3.2 | |
| Mussaenosidic acid (8) | 0.0 ± 2.3 | 2.3 ± 2.2 | 5.1 ± 2.5 | 1.1 ± 2.9 | −2.4 ± 1.8 | |
| Geniposidic acid (9) | 0.0 ± 2.3 | −2.8 ± 1.0 | −0.5 ± 1.2 | −0.7 ± 1.3 | 1.6 ± 1.4 | |
| 8-Epideoxyloganic acid (10) | 0.0 ± 1.1 | 3.9 ± 1.0 | 2.0 ± 0.9 | 0.9 ± 0.3 | 5.2 ± 1.3 | |
| Gluroside (12) | 0.0 ± 4.0 | 0.7 ± 2.0 | −3.5 ± 1.4 | −0.8 ± 4.5 | −0.4 ± 0.7 | |
| Antirrhide (13) | 0.0 ± 2.1 | −5.6 ± 1.0 | 2.6 ± 1.8 | 1.4 ± 3.7 | 15.2 ± 4.8b) | |
| Ajugol (14) | 0.0 ± 0.9 | 3.5 ± 1.4 | 2.4 ± 1.7 | 4.5 ± 1.7 | 4.1 ± 1.0 | |
| Bartsioside (15) | 0.0 ± 0.3 | 2.9 ± 1.3 | 1.2 ± 0.7 | −1.4 ± 1.5 | 10.4 ± 1.5b) | |
| 6-Deoxycatalpol (16) | 0.0 ± 2.0 | −2.4 ± 2.8 | −3.1 ± 2.2 | 0.7 ± 3.7 | 2.4 ± 3.4 | |
| Argyol (18) | 0.0 ± 4.3 | −5.9 ± 1.9 | 3.4 ± 2.5 | 5.7 ± 4.7 | 28.7 ± 4.6b) | |
| Cistanin (19) | 0.0 ± 2.1 | 0.4 ± 1.2 | −1.1 ± 2.3 | 4.6 ± 4.3 | 16.8 ± 4.6b) | |
| Cistanchlorin (20) | 0.0 ± 1.2 | 9.4 ± 4.4 | 8.5 ± 2.1 | 11.3 ± 0.8a) | 32.8 ± 3.0b) | |
| Kankanoside E (21) | 0.0 ± 2.8 | 3.4 ± 3.6 | 2.6 ± 3.3 | −0.5 ± 3.1 | 7.5 ± 2.3 | |
| 24 | 0.0 ± 2.5 | 3.7 ± 2.4 | 6.2 ± 4.0 | 5.7 ± 2.0 | 22.3 ± 3.8b) | |
| Echinacoside (28) | 0.0 ± 2.1 | 32.8 ± 1.4b) | 46.7 ± 4.3b) | 67.7 ± 1.7b) | 10.2 | |
| Acteoside (29) | 0.0 ± 2.4 | 40.9 ± 1.3b) | 71.8 ± 2.3b) | 119.2 ± 5.4b) | 4.6 | |
| Kankanoside G (31) | 0.0 ± 3.0 | 12.6 ± 3.6a) | 33.3 ± 3.3b) | 72.7 ± 4.1b) | 14.8 | |
| Kankanoside H1 (32) | 0.0 ± 1.8 | 8.7 ± 3.2 | 16.4 ± 4.2a) | 20.4 ± 2.2b) | 34.0 ± 2.4b) | |
| Kankanoside H2 (33) | 0.0 ± 0.6 | 4.4 ± 1.1 | 11.6 ± 1.3b) | 18.2 ± 1.9b) | 26.3 ± 0.9b) | |
| Kankanoside I (34) | 0.0 ± 0.6 | 3.9 ± 0.6 | 13.6 ± 0.3b) | 25.9 ± 1.7b) | 27.7 ± 2.5b) | |
| Isoacteoside (39) | 0.0 ± 4.4 | 43.7 ± 2.1b) | 57.3 ± 2.2b) | 101.2 ± 5.9b) | 5.3 | |
| 2′-Acetylacteoside (41) | 0.0 ± 1.9 | 41.9 ± 3.2b) | 58.4 ± 5.3b) | 95.2 ± 3.2b) | 4.8 | |
| Tubuloside A (43) | 0.0 ± 3.7 | 31.1 ± 1.6b) | 50.2 ± 4.6b) | 74.6 ± 0.9b) | 8.6 | |
| Cistantubuloside B1 (44) | 0.0 ± 1.0 | 3.1 ± 1.2 | 10.3 ± 1.7b) | 18.5 ± 1.6b) | 31.2 ± 2.7b) | |
| Wiedemanninoside C (47) | 0.0 ± 0.5 | 4.5 ± 1.7 | 11.5 ± 0.9b) | 20.6 ± 2.6b) | 39.4 ± 2.8b) | |
| Tubuloside B (48) | 0.0 ± 4.4 | 8.6 ± 2.3 | 33.6 ± 4.5b) | 75.4 ± 2.8b) | 14.6 | |
| Syringalide A 3′-O-Rha (49) | 0.0 ± 1.3 | 9.7 ± 0.7 | 21.4 ± 1.5b) | 35.7 ± 4.0b) | 55.7 ± 6.1b) | 71.2 |
| Cistantubuloside A (50) | 0.0 ± 1.9 | 3.0 ± 1.5 | 8.2 ± 3.4 | 17.0 ± 4.1b) | 15.3 ± 3.4b) | |
| Salidroside (52) | 0.0 ± 1.8 | 0.9 ± 0.6 | 1.4 ± 1.4 | −0.7 ± 1.8 | 0.2 ± 1.3 | |
| Kankanose (56) | 0.0 ± 2.8 | −4.9 ± 1.3 | −1.3 ± 2.9 | −7.9 ± 2.1 | −2.8 ± 2.8 | |
| Cistanoside F (57) | 0.0 ± 1.5 | 2.0 ± 0.7 | 4.0 ± 2.6 | 7.7 ± 3.9 | 21.2 ± 0.8a) | |
| (+)-Pinoresinol Glc (61) | 0.0 ± 3.4 | −8.9 ± 2.8 | 3.6 ± 3.9 | 25.9 ± 3.4b) | 77.4 ± 4.6b) | 48.5 |
| (+)-Syringaresinol Glc (64) | 0.0 ± 3.6 | −10.6 ± 2.9 | −2.1 ± 3.2 | 9.2 ± 4.2 | 50.6 ± 2.6b) | 98.4 |
| (2R,3R)-Butane-2,3-diol 2-O-Glc (65) | 0.0 ± 3.1 | −14.6 ± 3.1 | −13.8 ± 3.0 | −17.4 ± 4.9 | −6.6 ± 6.5 | |
| Ethyl Glc (67) | 0.0 ± 1.6 | −5.6 ± 1.6 | −6.8 ± 2.6 | −7.7 ± 2.4 | 3.5 ± 3.0 | |
| 3-Methylbutan-1-ol Glc (68) | 0.0 ± 2.4 | 4.3 ± 2.6 | −0.6 ± 1.7 | −1.2 ± 0.8 | −6.4 ± 0.2 | |
| Curcumin105–108,114–116) | 0.0 ± 3.7 | 0.1 ± 3.8 | 1.1 ± 2.2 | −17.7 ± 1.3 | −44.3 ± 0.3 | |
| Silybinc),115,116) | 0.0 ± 0.3 | 4.8 ± 1.1 | 7.7 ± 0.7 | 45.2 ± 8.8b) | 77.0 ± 5.5b) | 38.8 |
Each value represents the mean ± S.E.M. (N = 4). Significantly different from control: a) p < 0.05; b) p < 0.01. c) Commercial silybin was purchased from Funakoshi Co., Ltd. (Tokyo, Japan). Reproduced in part with permission from Bioorg. Med. Chem., 18, 1882–1890. Copyright [2010]. Elsevier.
Next, effects of the methanol extract from the stems of C. tubulosa and its principal phenylethanoid glycosides, echinacoside (28), acteoside (29), and isoacteoside (39), on NO and TNF-α production, as markers of macrophage activation in LPS-activated mouse peritoneal macrophages128) were examined. The methanol extract and these constituents (28, 29, and 39) showed neither NO nor TNF-α production inhibitory activities (IC50 > 100 µM, data not shown).23) These finding led us to suggest that they did not affect the overproduction of NO and TNF-α from LPS-activated macrophages.
To clarify the effects on the sensitivities of hepatocytes to TNF-α, decrease in cell viability of a TNF-α-sensitive L929 cells induced by TNF-α were assessed. As shown in Table 5, several phenylethanoid glycosides, echinacoside (28, IC50 = 31.1 µM), acteoside (29, 17.8 µM), isoacteoside (39, 22.7 µM), 2′-acetylacteoside (41, 25.7 µM), tubuloside A (43, 23.2 µM), and cistantubuloside B1 (44, 21.4 µM) showed relatively strong activity, which was greater than that of silybin (60.4 µM).23) In addition, the following isolates were found to exert significant activity (p < 0.01): kankanoside A (1, inhibition: 16.3 ± 2.0% at 100 µM), mussaenosidic acid (8, 44.7 ± 8.7%), 8-epideoxyloganic acid (10, 10.7 ± 0.4%), 8-hydroxygeraniol 8-O-β-D-glucopyranoside (26, 21.3 ± 2.4%), tubuloside B (48, 39.2 ± 6.3%), syringalide A 3′-O-α-L-rhamnopyranoside (49, 22.2 ± 6.4%), cistantubuloside A (50, 11.2 ± 1.1%), and (+)-pinoresinol O-β-D-glucopyranoside (61, 22.3 ± 1.6%). Although their activities were weaker than those of the above-mentioned principal phenylethanoid glycosides, the main active constituents are considered to be echinacoside (28), acteoside (29), and isoacteoside (39), etc. The structural requirements of the phenylethanoid glycosides for their activity were: (i) the aglycone part was essential for activity [echinacoside (28) >> kankanose (56, >100 µM)]; (ii) the aglycone having the 3,4-dihydroxy group showed stronger activity than that having the 4-hydroxy group [echinacoside (28) > cistantubuloside A (50, >100 µM); acteoside (29) > syringalide A 3′-O-α-L-rhamnopyranoside (49, >100 µM); isoacteoside (39) > kankanoside G (31, >100 µM)]; (iii) the 6′-O-β-D-glucopyranosyl moiety reduced the activity [acteoside (29) > echinacoside (28); 2′-acetylacteoside (41) ≥ tubuloside A (43)]; (iv) the 8-O-β-D-glucopyranosyl part having the 4′-O-caffeoyl group showed stronger activity than that having the 6′-O-caffeoryl group [acteoside (29) > isoacteoside (39); 2′-acetylacteoside (41) > tubuloside B (48, >100 µM)]; and (v) introduction of the 2′-O-acetyl moiety reduced the activity [acteoside (29) and tubuloside B (48) > 2′-acetylacteoside (41)]. These requirements were similar to those mentioned above concerning the inhibitory effects on D-GalN-induced cytotoxicity in primary cultured mouse hepatocytes.
| Inhibition (%) | IC50 (µg/mL) | |||||
|---|---|---|---|---|---|---|
| 0 µg/mL | 3 µg/mL | 10 µg/mL | 30 µg/mL | 100 µg/mL | ||
| Methanol extract | 0.0 ± 1.4 | 17.6 ± 8.1 | 40.5 ± 5.3b) | 58.3 ± 4.6b) | 47.9 ± 4.4b) | 18.4 |
| Inhibition (%) | IC50 (µM) | |||||
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | ||
| Kankanoside A (1) | 0.0 ± 0.9 | 8.8 ± 2.7 | 15.5 ± 3.1b) | 17.2 ± 3.7b) | 16.3 ± 2.0b) | |
| Kankanoside B (2) | 0.0 ± 1.7 | −0.8 ± 1.1 | −1.8 ± 0.4 | −6.7 ± 0.7 | −11.5 ± 0.6 | |
| Mussaenosidic acid (8) | 0.0 ± 3.5 | 10.8 ± 6.9 | 26.5 ± 7.7a) | 72.0 ± 1.6b) | 44.7 ± 8.7b) | |
| Geniposidic acid (9) | 0.0 ± 2.5 | 2.3 ± 1.8 | 19.8 ± 3.9b) | 24.1 ± 1.5b) | −2.5 ± 3.4 | |
| 8-Epideoxyloganic acid (10) | 0.0 ± 1.5 | 8.0 ± 2.8 | 4.3 ± 1.9 | 9.7 ± 2.2a) | 10.7 ± 0.4b) | |
| Gluroside (12) | 0.0 ± 1.6 | 2.1 ± 1.3 | −2.7 ± 4.4 | −15.8 ± 2.2 | −4.8 ± 3.3 | |
| Antirrhide (13) | 0.0 ± 5.7 | −4.4 ± 8.3 | −5.5 ± 7.2 | −9.7 ± 3.0 | −8.0 ± 3.5 | |
| Ajugol (14) | 0.0 ± 2.7 | 2.7 ± 8.1 | −3.2 ± 2.3 | −2.3 ± 5.4 | −4.0 ± 1.6 | |
| Bartsioside (15) | 0.0 ± 0.4 | 1.4 ± 2.0 | 5.5 ± 2.2 | 6.1 ± 2.0 | 1.1 ± 1.6 | |
| 6-Deoxycatalpol (16) | 0.0 ± 1.2 | 1.3 ± 2.1 | 1.1 ± 1.9 | −1.9 ± 2.4 | 0.2 ± 3.5 | |
| Argyol (18) | 0.0 ± 8.1 | −0.5 ± 10.3 | 3.5 ± 9.5 | −10.7 ± 5.7 | −6.8 ± 7.7 | |
| Cistanin (19) | 0.0 ± 3.8 | 10.2 ± 3.6 | 11.9 ± 6.4 | 12.6 ± 5.4 | −7.7 ± 4.7 | |
| Cistanchlorin (20) | 0.0 ± 2.6 | 9.8 ± 2.8 | 13.7 ± 5.3 | −5.7 ± 4.2 | −3.6 ± 7.1 | |
| Kankanoside E (21) | 0.0 ± 3.7 | −5.4 ± 7.2 | 15.8 ± 2.9 | 19.3 ± 2.6 | 2.1 ± 12.1 | |
| 24 | 0.0 ± 1.6 | 5.0 ± 3.0 | 3.3 ± 5.0 | 4.2 ± 2.7 | 2.8 ± 2.7 | |
| 25 | 0.0 ± 1.1 | −2.5 ± 0.5 | −1.5 ± 1.6 | −2.8 ± 2.7 | −8.1 ± 2.4 | |
| 8-Hydroxygeraniol 8-O-Glc (26) | 0.0 ± 3.1 | 3.6 ± 6.0 | 4.4 ± 7.9 | 9.9 ± 3.3 | 21.3 ± 2.4b) | |
| Echinacoside (28) | 0.0 ± 4.8 | 5.2 ± 3.5 | 22.5 ± 1.6b) | 45.7 ± 6.0b) | 80.4 ± 4.5b) | 31.1 |
| Acteoside (29) | 0.0 ± 1.1 | 16.4 ± 1.3a) | 24.1 ± 4.6b) | 58.4 ± 2.5b) | 91.9 ± 5.3b) | 17.8 |
| Kankanoside G (31) | 0.0 ± 2.8 | 1.3 ± 0.9 | 4.7 ± 0.5 | 3.1 ± 2.6 | 2.9 ± 1.4 | |
| Isoacteoside (39) | 0.0 ± 1.2 | −4.6 ± 3.5 | 19.0 ± 2.6 | 61.9 ± 5.9b) | 102.4 ± 8.7b) | 22.7 |
| 2′-Acetylacteoside (41) | 0.0 ± 3.1 | 2.3 ± 5.0 | 8.9 ± 6.6 | 64.1 ± 4.9b) | 107.3 ± 10.4b) | 25.7 |
| Tubuloside A (43) | 0.0 ± 2.4 | 14.7 ± 4.6a) | 36.2 ± 4.8b) | 55.2 ± 2.8b) | 101.9 ± 2.2b) | 23.2 |
| Cistantubuloside B1 (44) | 0.0 ± 3.9 | −14.7 ± 17.2 | 31.0 ± 4.4b) | 32.8 ± 10.8b) | 122.7 ± 13.7b) | 21.4 |
| Tubuloside B (48) | 0.0 ± 4.9 | 10.7 ± 4.7 | 13.4 ± 4.7 | 36.4 ± 13.3a) | 39.2 ± 6.3b) | |
| Syringalide A 3′-O-Rha (49) | 0.0 ± 2.9 | 4.5 ± 1.0 | 4.6 ± 1.4 | 13.3 ± 3.3 | 22.2 ± 6.4b) | |
| Cistantubuloside A (50) | 0.0 ± 2.3 | 2.8 ± 1.2 | 3.6 ± 0.5 | 4.6 ± 1.6 | 11.2 ± 1.1a) | |
| Salidroside (52) | 0.0 ± 6.1 | −1.2 ± 7.9 | −8.3 ± 10.5 | −5.4 ± 5.1 | −1.0 ± 4.8 | |
| Campneoside I (54) | 0.0 ± 2.0 | 7.7 ± 2.9 | −8.8 ± 8.5 | 1.9 ± 5.8 | 7.5 ± 3.1 | |
| Kankanose (56) | 0.0 ± 1.9 | −1.1 ± 1.2 | 2.2 ± 1.8 | 1.3 ± 1.8 | 0.8 ± 0.1 | |
| (+)-Pinoresinol Glc (61) | 0.0 ± 1.3 | 4.1 ± 3.3 | 10.1 ± 5.8 | 13.9 ± 3.3a) | 22.3 ± 1.6b) | |
| (+)-Syringaresinol Glc (64) | 0.0 ± 0.8 | 4.5 ± 4.5 | −1.4 ± 2.9 | −0.4 ± 2.0 | −2.3 ± 4.3 | |
| (2R,3R)-Butane-2,3-diol 2-O-Glc (65) | 0.0 ± 0.8 | −1.3 ± 1.3 | −2.0 ± 1.6 | −5.0 ± 0.4 | −5.8 ± 1.6 | |
| Ethyl Glc (67) | 0.0 ± 1.8 | −2.7 ± 1.3 | 2.4 ± 1.8 | −8.0 ± 6.0 | −3.0 ± 1.7 | |
| 3-Methylbutan-1-ol Glc (68) | 0.0 ± 4.4 | 6.4 ± 3.5 | 6.0 ± 9.5 | 3.6 ± 2.9 | 8.5 ± 0.8 | |
| Silybinc),115,116) | 0.0 ± 2.6 | 5.3 ± 2.8 | 22.0 ± 3.8b) | 48.0 ± 4.1b) | 50.8 ± 3.9b) | 60.4 |
Each value represents the mean ± S.E.M. (N = 4). Significantly different from control: a) p < 0.05; b) p < 0.01. c) Commercial silybin was purchased from Funakoshi Co., Ltd. (Tokyo, Japan). Reproduced in part with permission from Chem. Pharm. Bull., 58, 1403–1407. Copyright [2010]. The Pharmaceutical Society of Japan and from Bioorg. Med. Chem., 18, 1882–1890. Copyright [2010]. Elsevier.
These findings suggest that the possible mechanisms of action for the hepatoprotective effects of the phentyethanoid glycosides from the stems of C. tubulosa are: (i) decreasing of D-GalN-induced cytotoxicity [echinacoside (28), acteoside (29), kankanoside G (31), isoacteoside (39), 2′-acetylacteoside (41), and tubulosides A (43) and B (48)], and (ii) decreasing of TNF-α-induced cytotoxicity [echinacoside (28), acteoside (29), isoacteoside (39), 2′-acetylacteoside (41), tubuloside A (43), and cistantubuloside B1 (44)]. Among them, the principal phenylethanoid glycosides, echinacoside (28), acteoside (29), and isoacteoside (39), were found to inhibit the increase in serum sAST and sALT levels at doses of 25–100 mg/kg, per os (p.o.) against D-GalN/LPS-induced acute liver injury in mice (vide ante), and those inhibitory effects were suggested to be dependent on the decreasing cytotoxicity caused by D-GalN and reduction of sensitivity of hepatocytes to TNF-α. As summarized in Fig. 3, these results suggest that the mechanisms of action are different to those of not only curcumin and silybin but also of piperine111–113) and trans-resveratrol,116) which were investigated as hepatoprotective natural products in our previous study.
Diabetes is characterized by a high incidence of cardiovascular disease, and poor control of hyperglycemia appears to play a significant role in the development of cardiovascular disease in diabetes. There has been increasing evidence that the postprandial state is an important contributing factor to the development of atherosclerosis. In diabetes, the postprandial phase is characterized by a rapid and large increase in blood glucose levels, and the possibility that these postprandial hyperglycemic spikes may be relevant to the pathophysiology of late diabetes complications is recently receiving much attention. Therefore improving postprandial hyperglycemia may form part of the strategy for the prevention and management of cardiovascular disease in diabetes.136) We previously reported that several antidiabetogenic therapeutic candidates were obtained from natural resources such as Kochia scoparia,137) Borassus flabellifer,138) Solanum lycocarpum,139) Sinocrassula indica,140) Shorea roxburghii,141) Helichrysum arenarium,142) and Salacia reticulata, S. oblonga, and S. chinensis143–148) evaluated by postprandial hyperglycemia in sugar-loaded rats and/or mice models. In these experiments the effects of the principal phenylethanoid glycosides from the stems of C. tubulosa, echinacoside (28) and acteoside (29), on postprandial increase in blood glucose levels in starch-loaded mice were examined. As shown in Table 6, both phenylethanoid glycosides (28 and 29) significantly suppressed increases in blood glucose levels at doses of 250–500 mg/kg, p.o.26)
| Treatment | Dose (mg/kg, p.o.) | N | Plasma glucose (mg/dL) | AUC (h·mg/dL) | |||
|---|---|---|---|---|---|---|---|
| 0 h | 0.5 h | 1 h | 2 h | ||||
| Control | — | 9 | 88.9 ± 3.8 | 246.9 ± 10.5 | 210.0 ± 9.8 | 174.8 ± 8.2 | 390.6 ± 8.8 |
| Echinacoside (28) | 250 | 5 | 87.7 ± 7.0 | 255.0 ± 11.6 | 179.5 ± 4.1a) | 148.1 ± 8.2 | 358.0 ± 12.3a) |
| 500 | 5 | 81.4 ± 7.1 | 227.5 ± 5.2 | 175.7 ± 3.0b) | 135.7 ± 7.9b) | 333.7 ± 5.6b) | |
| Acteoside (29) | 250 | 5 | 88.1 ± 4.2 | 235.4 ± 4.7 | 195.8 ± 4.3 | 164.6 ± 10.0 | 368.8 ± 8.6 |
| 500 | 5 | 87.0 ± 5.1 | 222.3 ± 8.0 | 178.5 ± 3.3a) | 143.6 ± 5.3a) | 338.5 ± 4.0b) | |
| Control | — | 9 | 100.4 ± 7.2 | 216.0 ± 8.4 | 197.3 ± 7.1 | 172.8 ± 5.1 | 367.5 ± 12.2 |
| Acarbosec) | 12.5 | 7 | 103.6 ± 7.9 | 160.6 ± 7.4b) | 190.5 ± 6.7 | 201.2 ± 8.8a) | 349.7 ± 12.9 |
| 25 | 99.2 ± 3.4 | 136.1 ± 3.8b) | 149.9 ± 4.2b) | 182.0 ± 10.0 | 296.3 ± 8.2b) | ||
Each value represents the mean ± S.E.M. Significantly different from control: a) p < 0.05; b) p < 0.01. c) Commercial acarbose was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Reproduced with permission from J. Nat. Med., 68, 561–566. Copyright [2014]. Springer.
Next, the effects of echinacoside (28) and acteoside (29) on glucose tolerance were evaluated in starch-loaded mice after 2 weeks of continuous administration. As shown in Table 7, both phenylethanoid glycosides (28 and 29) were found significantly to improve glucose tolerance without significant changes in body weight and food intake (data not shown). These results suggest that echinacoside (28) and acteoside (29) may be effective in suppressing postprandial glucose elevation and improving glucose tolerance.26)
| Treatment | Dose (mg/kg/d, p.o.) | N | Plasma glucose (mg/dL) | AUC (h·mg/dL) | |||
|---|---|---|---|---|---|---|---|
| 0 h | 0.5 h | 1 h | 2 h | ||||
| Control | — | 9 | 99.2 ± 8.5 | 203.9 ± 8.5 | 172.4 ± 5.4 | 146.8 ± 5.3 | 329.5 ± 9.3 |
| Echinacoside (28) | 125 | 5 | 82.2 ± 4.3 | 182.9 ± 10.9 | 143.7 ± 8.4a) | 122.1 ± 2.7b) | 280.9 ± 10.6b) |
| 250 | 5 | 84.0 ± 4.9 | 185.7 ± 10.2 | 147.1 ± 7.7a) | 124.4 ± 2.3b) | 286.4 ± 10.8a) | |
| Acteoside (29) | 125 | 5 | 86.1 ± 5.6 | 210.7 ± 10.5 | 179.5 ± 8.4 | 145.0 ± 4.0 | 334.0 ± 12.7 |
| 250 | 5 | 79.4 ± 2.3 | 170.6 ± 6.9 | 144.4 ± 6.4a) | 107.1 ± 5.0b) | 267.0 ± 10.7b) | |
Values represent the means ± S.E.M. Significantly different from control group: a) p < 0.05; b) p < 0.01. Reproduced with permission from J. Nat. Med., 68, 561–566. Copyright [2014]. Springer.
To characterize the mode of action of the suppressing postprandial glucose elevation activity, enzymatic inhibitory effects of the isolates from the stems of C. tubulosa on rat small intestinal α-glucosidases such as maltase and sucrase were evaluated. Several phenylethanoid glycosides, echinacoside (28, IC50 = 149 and 174 µM against maltase and sucrase, respectively), acteoside (29, 188 and 152 µM), kankanosides J1 (35, 130 and 242 µM) and J2 (36, 131 and 228 µM), isoacteoside (39, 70.4 and 152 µM), and tubulosides A (43, 200 and 220 µM) and B (48, 88.2 and 175 µM) showed enzymatic inhibitory activities, whereas none of the isolates showed activities at concentrations of up to 300 µM26) (Table 8). Furthermore, their activities were far less than those of the positive agents such as clinically used ararbose (1.7 and 1.5 µM)147,149–151) and one of the most potent naturally occurring inhibitors, salacinol (6.0 and 1.3 µM),147,149,151) and neokotalanol (1.6 and 1.5 µM).147,149,150) As shown in Table 9, enzymatic inhibitory activities of echinacoside (28), acteoside (29), isoacteoside (39), and tubulosides A (43) and B (48) on different α-glucosidases originating from yeast (Saccharomyces cerevisiae), bacteria (Bacillus stearothermophilus), and human small intestine were also observed. As for the IC50 values for human small intestinal maltase, these phanylethanoids (28, 29, 39, 43, 48, IC50 = 117–163 µM) were far less active than acarbose (15.2 µM).26) On the basis of the above-mentioned evidence, contribution of the α-glucosidase inhibitory activity of the phenylethanoid glycosides was found to limit as the mode of action of the suppressing effects on postprandial glucose elevation and the glucose tolerance-improving effects. As the possible mode of action, we recently reported that inhibitory effects of echinacoside (28) and acteoside (29) on sodium-dependent glucose co-transporter 1-mediated glucose uptake were observed.152) More detailed mode of action should be studied further.
| Rat α-glucosidase IC50 (µM) | Aldose reductase IC50 (µM) | ||
|---|---|---|---|
| Maltase | Sucrase | ||
| Kankanoside A (1) | >300 (2.5)a) | >300 (0.8)a) | >10 (3.9)b) |
| Kankanoside B (2) | >300 (7.1)a) | >300 (7.4)a) | >10 (18.6)b) |
| Kankanoside L (5) | >300 (0.7)a) | >300 (−1.4)a) | >10 (18.0)b) |
| Kankanoside N (7) | >300 (−4.1)a) | >300 (−7.7)a) | >10 (14.9)b) |
| Mussaenosidic acid (8) | >300 (−0.3)a) | >300 (0.7)a) | >10 (17.2)b) |
| Geniposidic acid (9) | >300 (−2.2)a) | >300 (0.8)a) | >10 (18.5)b) |
| 8-Epideoxyloganic acid (10) | >300 (−11.0)a) | >300 (−6.8)a) | >10 (25.0)b) |
| Gluroside (12) | >300 (−2.4)a) | >300 (2.5)a) | >10 (19.2)b) |
| Antirrhide (13) | >300 (5.6)a) | >300 (11.5)a) | >10 (11.5)b) |
| Bartsioside (15) | >300 (−3.8)a) | >300 (−7.3)a) | >10 (27.0)b) |
| 6-Deoxycatalpol (16) | >300 (−2.6)a) | >300 (1.9)a) | >10 (−1.1)b) |
| Argyol (18) | >300 (2.8)a) | >300 (7.3)a) | >10 (12.1)b) |
| Cistanin (19) | >300 (−2.2)a) | >300 (4.1)a) | >10 (2.7)b) |
| Cistanchlorin (20) | >300 (0.4)a) | >300 (2.3)a) | >10 (25.3)b) |
| Kankanoside E (21) | >300 (−3.9)a) | >300 (−1.8)a) | >10 (11.7)b) |
| Kankanoside O (22) | >300 (−2.9)a) | >300 (−6.5)a) | |
| 24 | >300 (−8.9)a) | >300 (−6.7)a) | >10 (9.4)b) |
| 25 | >300 (−12.6)a) | >300 (−10.2)a) | >10 (16.6)b) |
| 8-Hydroxygeraniol 8-O-Glc (26) | >300 (−13.8)a) | >300 (−10.3)a) | >10 (13.9)b) |
| Echinacoside (28) | 149 | 174 | 3.1 |
| Acteoside (29) | 188 | 152 | 1.2 |
| Kankanoside H1 (32) | >300 (37.1)a) | >300 (32.5)a) | >10 (33.9)b) |
| Kankanoside H2 (33) | >300 (4.6)a) | >300 (3.1)a) | |
| Kankanoside I (34) | >300 (27.1)a) | >300 (26.2)a) | >10 (33.9)b) |
| Kankanoside J1 (35) | 130 | 242 | 9.3 |
| Kankanoside J2 (36) | 131 | 228 | >10 (39.6)b) |
| Kankanoside K1 (37) | >300 (44.9)a) | >300 (38.7)a) | >10 (41.7)b) |
| Kankanoside K2 (38) | >300 (47.6)a) | >300 (38.7)a) | >10 (38.2)b) |
| Isoacteoside (39) | 70.4 | 152 | 4.6 |
| 2′-Acetylacteoside (41) | >300 (47.2)a) | 277 | 0.071 |
| Tubuloside A (43) | 200 | 220 | 8.8 |
| Wiedemanninoside C (47) | >300 (46.2)a) | >300 (43.5)a) | >10 (37.8)b) |
| Tubuloside B (48) | 88.2 | 175 | 4.0 |
| Syringalide A 3′-O-Rha (49) | >300 (32.7)a) | >300 (27.3)a) | 1.1 |
| Cistantubuloside A (50) | >300 (20.5)a) | >300 (27.2)a) | >10 (29.4)b) |
| Salidroside (52) | >300 (5.9)a) | >300 (8.1)a) | >10 (12.3)b) |
| Campneoside I (53) | >300 (27.0)a) | >300 (38.8)a) | 0.53 |
| Syringin (59) | >300 (−0.7)a) | >300 (−2.2)a) | >10 (25.6)b) |
| (+)-Pinoresinol Glc (61) | >300 (−3.0)a) | >300 (−7.9)a) | >10 (6.5)b) |
| Isoeucommin A (63) | >300 (3.2)a) | >300 (13.0)a) | >10 (8.7)b) |
| (+)-Syringaresinol Glc (64) | >300 (10.4)a) | >300 (24.8)a) | >10 (4.0)b) |
| Ethyl Glc (67) | >10 (11.1)b) | ||
| 3-Methylbutan-1-ol Glc (68) | >10 (3.3)b) | ||
| Acarbosec),147,149–151) | 1.7 | 1.5 | |
| Salacinol147,149,151) | 6.0 | 1.3 | |
| Neokotalanol147,149,150) | 1.6 | 1.5 | |
| Epalrestat c),154–161) | 0.072 | ||
Each value represents the mean of 2–4 experiments. Values in parentheses present inhibition % at a) 300 µM (for α-glucosidases) or b) 10 µM (for aldose reductase). c) Commercial acarbose and epalrestat were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Reproduced in part with permission from J. Nat. Med., 68, 561–566. Copyright [2014]. Springer.
| Enzyme origin | Yeast (Saccharomyces cerevisiae) IC50 (µM) | Bacteria (Bacillus stearothemophilus) IC50 (µM) | Human small intestine IC50 (µM) | ||
|---|---|---|---|---|---|
| Maltase | Sucrase | Maltase | Sucrase | Maltase | |
| Echinacoside (28) | >300 (45.0)a) | 146 | >300 (44.3)a) | 144 | 125 |
| Acteoside (29) | 193 | 127 | >300 (23.2)a) | 118 | 154 |
| Isoacteoside (39) | >300 (47.0)a) | 130 | >300 (18.5)a) | 139 | 117 |
| Tubuloside A (43) | 276 | 167 | >300 (25.4)a) | 142 | 163 |
| Tubuloside B (48) | 156 | 131 | 88.2 | 89.7 | 139 |
| Acarboseb) | >300 (21.3)a) | >300 (33.6)a) | 0.20 | 0.021 | 15.2 |
Each value represents the mean of 2–4 experiments. Values in parentheses present inhibition % at a) 300 µM. b) Commercial acarbose was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
Aldose reductase as a key enzyme in the polyol pathway catalyzes the reduction of glucose to sorbitol. In normal tissue, aldose reductase has low substrate affinity to glucose, so that the conversion of glucose to sorbitol is little catalyzed. However, in diabetes mellitus, the increased availability of glucose in insulin-insensitive tissues such as lens, nerve, and retina leads to the increased formation of sorbitol through the polyol pathway. Sorbitol does not readily diffuse across cell membranes and the intracellular accumulation of sorbitol has been implicated in the chronic complications of diabetes such as cataract, neuropathy, and retinopathy. These findings suggest that an aldose reductase inhibitor may have the capacity of preventing and/or treating several diabetic complications.153) As potent aldose reductase inhibitors from natural resources, we identified several flavonoids,154–160) stilbenoids,141,154) terpenoids,161) and quinic acid derivatives.159) As shown in Table 8, several phenylethanoid glycosides, echinacoside (28, IC50 = 3.1 µM), acteoside (29, 1.2 µM), kankanoside J1 (35, 9.3 µM), isoacteoside (39, 4.6 µM), 2′-acetylacteoside (41, 0.071 µM), tubulosides A (43, 8.8 µM) and B (48, 4.0 µM), syringalide A 3′-O-α-L-rhamnopyranoside (49, 1.1 µM), and campneoside I (53, 0.53 µM) showed rat lens aldose reductase inhibitory activity. Especially, 2′-acetylacteoside (41) was the most potent and equivalent to that of epalrestat (0.072 µM), a clinically used aldose reductase inhibitor.
In this review, our recent studies on chemical constituents from the stems of C. tubulosa as well as their bioactivities such as vasorelaxtant, hepatoprotective, and glucose tolerance-improving activities have been summarized.
As for the chemical constituents, 20 ididoids (1–20) including eight new ones, kankanosides A–D (1–4), L–N (5–7), and kankanol (17), seven aliphatic monoterpenoid glycosides (21–27) including three new ones, kankanosides E (21), O (22), and P (23), 28 phenylethanoid glycosides (28–55) including nine new ones, kankanosides F (30), G (31), H1 (32), H2 (33), I (34), J1 (35), J2 (36), K1 (37), and K2 (38), two sugar esters (56 and 57) including a new one, kankanose (57), three phenylpropanoid glycosides (58–60), four lignin glycosides (61–64), four alkyl glycosides (65–68), and sugar alcohol (69) were isolated from the methanol extract. The vasorelaxant active principles, several phenylethanoid glycosides such as echinacoside (28) and acteoside (29), and the related isolates, kankanoside F (30), isoacteoside (39), kankanose (56), and cistanoside F (57), were identified. They elicited NA-induced contractions in isolated rat thoracic aorta, but did not inhibit high K+-induced contractions. These findings suggest that these active constituents inhibit the contractions via receptor-operated calcium channel, but not via voltage-dependent calcium channel. Especially, echinacoside (28) and acteoside (29) significantly inhibited NA-induced contractions, time- and concentration-dependently (10–100 µM), in a different manner from that of prazosin. The principal phenylethanoid glycosides, echinacoside (28), acteoside (29), and isoacteoside (39), exhibited hepatoprotective effect against D-GalN/LPS-induced acute liver injury in mice at doses of 25–100 mg.kg, p.o. To characterize the mechanisms of action, the isolates were examined in in vitro studies assessing their effects on (i) D-GalN-induced cytotoxicity in primary cultured mouse hepatocytes: (ii) LPS-induced NO and/or TNF-α production in mouse peritoneal macrophages: and (iii) TNF-α-induced cytotoxicity in L929 cells. The mechanisms of action of these principal phenylethanoid glycosides (28, 29, and 39) were dependent on decreasing cytotoxicity caused by D-GalN and reduction of sensitivity of hepatocytes to TNF-α. Furthermore, echinacoside (28) and acteoside (29) were found to suppress postprandial glucose elevation and improve glucose tolerance by single-dose and 2-week administration treated starch-loaded mice models. Furthermore, one of the phenylethanoid glycoside constituents, 2′-acetylacteoside (41, IC50 = 0.071 µM) showed potent aldose reductase inhibitory activity, which was equivalent to that of a clinically used epalrestat (0.072 µM). In addition, several structural requirements of the phenylethanoid glycosides for their above-mentioned biological activities were characterized. Based on the evidence collected, C. tubulosa and its constituents, in particular echinacoside (28) and acteoside (29), may be useful for the treatment of various lifestyle diseases such as hypertension, diabetes, and hepatitis, etc.
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018, Japan (S1411037, T.M.), as well as the JSPS KAKENHI, Japan [Grant Numbers 18K06726 (T.M.), 18K06739 (K.N.), and 16K08313 (O.M.)].
The authors declare no conflict of interest.