Experimental
Melting points were determined using a micro melting point apparatus (Yanaco MP-S3) without correction. IR spectra were measured by a Shimadzu FTIR-8100 IR spectrophotometer. Low- and high-resolution mass spectra (LR-MS and HR-MS) were obtained by a JEOL JMS HX-110 double-focusing model equipped with an FAB ion source interfaced with a JEOL JMA-DA 7000 data system. lH- and 13C-NMR spectra were obtained by JEOL JNM A-500. Chemical shifts were expressed in δ ppm downfield from an internal TMS signal for lH-NMR and the carbon signal of the corresponding solvent [CDCl3 (77.00 ppm), DMSO-d6 (39.50 ppm), and THF-d8 (68.60 ppm)] for 13C-NMR. The abbreviations qu=quintet, dm=double multiplets and tm=triple multiplets are used for the multiplicity of lH-NMR data, respectively. The signal assignments were confirmed by two-dimensional (2D)-NMR analyses: lH–lH 2D correlation spectroscopy (COSY), lH–l3C heteronuclear multiple-quantum coherence (HMQC), lH–l3C heteronuclear multiple-bond connectivity (HMBC). Microanalyses were performed with a Yanaco MT-6 CHN corder. Routine monitoring of reactions was carried out using precoated Kieselgel 60F254 plates (E. Merck). Centrifugal or flash column chromatography was performed on silica gel (Able-Biott or Kanto 60N) with a UV detector. Commercially available starting materials were used without further purification, and dry solvents were used in all reactions except for Entry 8.
General Procedure for the Preparation of Alkoxy-amino-1,3,5-triazine Derivatives (Method A): Example: Preparation of 1,1′-[6-(2-Methoxyethoxy)-1,3,5-triazine-2,4-diyl]bis(4-piperidinemethanol) (6app) (Entry 1): (Step 1)To a solution of 2,4,6-trichlorotriazine (TAZ: cyanuric chloride) (1, 922 mg, 5.0 mmol) and collidine (727 mg, 6.0 mmol) in dry acetone (10 mL) was added 2-methoxyethanol (aH, 457 mg, 6.0 mmol) at 0°C. After stirring for 1 h at 0°C, the resulting colorless precipitated collidine·HCl was removed by filtration and then the solvent was evaporated to afford a yellow oily residue. (Step 2) This material was dissolved in dry CH3CN (15 mL), and 4-piperidinemethanol (dH, 2.30 g 20.0 mmol) and N,N-diisopropylethylamine (DIPEA, 2.58 g, 20.0 mmol) were added, and the resulting mixture was stirred for 14 h at room temperature. After evaporation of the solvent, the residue was separated by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=93 : 6.5 : 0.5→85 : 14.5 : 0.5) to give 6app (1.17 g, 61% yield) as a yellow solid. Compounds 6wpp [4,6-bis[4-(hydroxymethyl)piperidin-1-yl]-1,3,5-triazin-2(1H)-one ] (253 mg, 16% yield) as a pale yellow solid and 7wap [4-[4-(hydroxymethyl)piperidin-1-yl]-6-(2-methoxyethoxy)-1,3,5-triazin-2(1H)-one] (173 mg, 12% yield) as a colorless opaque solid were also isolated in reaction products. Compound 7aap was also detected by TLC. Recrystallization from EtCN or EtOH gave an analytically pure product 6app or 6wpp.
6app: Colorless crystals, mp 112–113°C (EtCN). IR (KBr) cm−1: 3416 (OH of alcohol), 1573, 1503 (C=N), 1099 (C–N), 1253, 1099, 1032 (C–O of alcohol and ether). 1H-NMR (CDCl3) δ: 1.17 (4H, m, H3′β, 5′β), 1.77 (6H, dm, J=12.8 Hz, H3′α, 5′α, 4′α), 1.82 (2H, br s, OH), 2.80 (4H, tm, J=13.1 Hz, H2′β, 6′β), 3.40 (3H, s, OCH3), 3.49 (4H, d, J=6.1 Hz, H1), 3.71 (2H, t, J=5.2 Hz, H2‴), 4.42 (2H, t, J=5.2 Hz, H1‴), 4.76 (4H, br d, J=13.1 Hz, H2′α, 6′α). 13C-NMR (CDCl3) δ: 28.55 (C3′, 5′), 39.08 (C4′), 43.27 (C2′, 6′), 58.89 (OCH3), 65.05 (C1‴), 67.54 (C1), 70.60 (C2‴), 165.80 (C2″, 4″), 170.73 (C6″). Positive-ion FAB-MS m/z: 382 (M+H+). HR-FAB-MS m/z: 382.2455 (Calcd for C18H32N5O4: 382.2454). Anal. Calcd for C18H31N5O4: C, 56.67; H, 8.19; N, 18.36. Found: C, 56.60; H, 8.17; N, 18.40.
6wpp: Colorless crystals, mp 262–264°C (EtOH). IR (KBr) cm−1: 3425, 3286 (OH of alcohol), 2935 (NH), 1643, 1587, 1550 (C=O and C=N), 1263, 1043 (C–O of alcohol). 1H-NMR (DMSO-d6) δ: 1.05 (4H, m, H3′β, 5′β), 1.62 (2H, m, H4′), 1.67 (4H, br d, J=13.1 Hz, H3′α, 5′α,), 2.80 (4H, m, H2′β, 6′β), 3.25 (4H, dd, J=5.8, 5.5 Hz, H1), 4.43 (4H, dd, J=5.8, 5.5 Hz, H2′α, 6′α), 4.46 (2H, br s, OH), 10.27 (1H, br s, NH). 13C-NMR (DMSO-d6) δ: 28.25 (C3′, 5′), 38.27 (C4′), 43.26 (C2′, 6′), 65.42 (C1), 157.44 (C4″, 6″), 159.82 (C2″). Positive-ion FAB-MS m/z: 324 (M+H+). HR-FAB-MS m/z: 324.2030 (Calcd for C15H26N5O3: 324.2036). Anal. Calcd for C15H25N5O3·0.3H2O : C, 54.79; H, 7.85; N, 21.30. Found: C, 54.77; H, 7.76; N, 21.24.
7wap: mp 156–158°C. IR (KBr) cm−1: 3480 (OH of alcohol), 2922 (NH), 1667, 1618, 1559, 1517, 1444 (C=O and C=N), 1295, 1131, 1095 (C–O of alcohol and ether). 1H-NMR (CDCl3) δ: 0.88 (0.3H, br s, NH), 1.25 (2H, m, H3′β, 5′β), ca. 1.25 (0.7H, OH on C4″), 1.80 (3H, m, H3′α, 5′α, 4′), ca. 1.8 (1H, OH on C1), 2.90 (2H, t, J=13.1 Hz, H2′β, 6′β), 3.39 (3H, s, OCH3), 3.51 (2H, d, J=6.1 Hz, H1), 3.69 (2H, t, J=4.9 Hz, H2‴), 4.50 (2H, t, J=4.9 Hz, H1‴), 4.77 (2H, br d, J=13.1 Hz, H2′α, 6′α), 4.88 (1H, br s, OH). 13C-NMR (CDCl3) δ: 28.54 (C3′, 5′), 38.65 (C4′), 44.30 (C2′, 6′), 59.01 (OCH3), 66.92 (C1‴), 67.08 (C1), 69.89 (C2‴), 159.68 (C4″), 162.35 (C2″), 164.10 (C6″). Positive-ion FAB-MS m/z: 285 (M+H+). HR-FAB-MS m/z: 285.1573 (Calcd for C12H21N4O4: 285.1563). Anal. Calcd for C12H20N4O4·0.25H2O: C, 49.90; H, 7.15; N, 19.40. Found: C, 49.90; H, 7.32; N, 19.18.
Preparation of [1-[4-Chloro-6-(2-methoxyethoxy)-1,3,5-triazin-2-yl]piperidin-4-yl]methanol (8ap) and [1-[4-Chloro-6-[Ethyl(Isopropyl)amino]-1,3,5-triazin-2-yl]piperidin-4-yl]methanol (5rp) (Entry 3): (Step 1)To a solution of 1 (922 mg, 5.0 mmol) and DIPEA (2.58 g, 20.0 mmol) in dry THF (125 mL) was added aH (380 mg, 5.0 mmol) at 0°C under an N2 atmosphere with stirring. After stirring for 15 min at 0°C and for 10 min at room temperature, additional DIPEA (2.58 g, 20.0 mmol) and aH (380 mg, 5.0 mmol) were added, and then the reaction mixture was stirred for 16 h at room temperature. (Step 2) To this yellow reaction mixture was added pH (1.15 g 10.0 mmol), and the resulting mixture was stirred at 70°C for 1 h under an N2 atmosphere. After filtration of the separated amine hydrochloride (pH·HCl), the filtrate was evaporated and the residue was separated by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=95 : 4.7 : 0.3→93 : 6.6 : 0.4) to give 5rp (221 mg, 14%), 8ap (941 mg, 62%), 6app (253 mg, 13%), and 5pp7) (22 mg, 1%). An analytical sample of 8ap was obtained by recrystallization from EtOH–H2O.
8ap: Colorless crystals, mp 86–87°C (EtOH–H2O). IR (KBr) cm−1: 3416 (OH of alcohol), 1618, 1592 (C=N), 1090, 1055 (C–O of alcohol and ether), 794 (C–Cl). 1H-NMR (CDCl3) δ: 1.23 (2H, m, H3′β, 5′β), 1.57 (2H, br s, OH), 1.83 (3H, m, H3′α, 5′α, 4′α), 2.91 (2H, m, H2′β, 6′β), 3.41 (3H, s, OCH3), 3.53 (2H, d, J=6.1 Hz, H1), 3.71 (2H, t, J=4.9 Hz, H2‴), 4.48 (2H, t, J=4.9 Hz, H1‴), 4.78 (2H, m, H2′α, 6′α). 13C-NMR (CDCl3) δ: 28.42 (C3′ or 5′), 28.50 (C5′ or 3′), 38.72 (C4′), 43.83 (C2′ or 6′), 43.97 (C6′ or 2′), 59.05 (OCH3), 66.87 (C1‴), 67.22 (C1), 70.19 (C2‴), 165.11 (C2″), 170.54 (C4″), 170.89 (C6″). Positive-ion FAB-MS m/z: 303 (M+H+). HR-FAB-MS m/z: 303.1229 (Calcd for C12H20ClN4O3: 303.1224). Anal. Calcd for C12H19 Cl N4O3·0.1H2O: C, 47.32; H, 6.35; N, 18.40. Found: C, 47.23; H, 6.22; N, 18.39.
5rp: 1H-NMR (CDCl3) δ: 1.95 (11H, m, CH3×3, H3′β, 5′β), 1.62 (1H, br s, OH), 1.79 (3H, m, H4′α, 3′α, 5′α,), 2.82 (2H, m, H2′β, 6′β), 3.41 (2H, m, NCH2CH3), 3.52 (2H, d, J=6.1 Hz, H1), 4.74 (2H, m, H2′α, 6′α), 4.98 (1H, m, NCH<). 13C-NMR (CDCl3) δ: 14.26 (NCH2CH3), 20.47 (NCH–CH3), 28.55 (C3′, 5′), 36.61 (NCH2CH3), 39.01 (C4′), 43.42 (C2′, 6′), 46.06 (NCH–CH3), 67.54 (C1), 164.05, 164.18 (C2″, 4″), 169.12 (C6″). Positive-ion FAB-MS m/z: 314 (M+H+). HR-FAB-MS m/z: 314.1748 (Calcd for C14H25ClN5O: 314.1748).
[1-[4,6-Bis(2-methoxyethoxy)-1,3,5-triazin-2-yl]piperidin-4-yl]methanol (7aap) (Entry 5)These compounds were prepared by method A under the conditions shown in Table 1. Purification of the products by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=95 : 4.7 : 0.3) gave 7aap (60%), 6app (8%), and 6wpp (25%).
7aap: Pale yellow oil. IR (NaCl) cm−1: 3420 (OH of alcohol), 1584, 1526 (C=N), 1118, 1036 (C–O of alcohol and ether). 1H-NMR (CDCl3) δ: 1.19 (2H, m, H3′β, 5′β), 1.79 (3H, m, H3′α, 5′α, 4′α), 2.03 (1H, br s, OH), 2.86 (2H, tm, J=13.1 Hz, H2′β, 6′β), 3.40 (6H, s, OCH3), 3.50 (2H, d, J=6.1 Hz, H1), 3.71 (4H, t, J=4.9 Hz, H2‴), 4.47 (4H, t, J=4.9 Hz, H1‴), 4.79 (2H, dm, J=13.1 Hz, H2′α, 6′α). 13C-NMR (CDCl3) δ: 28.49 (C3′, 5′), 38.82 (C4′), 43.56 (C2′, 6′), 58.87 (OCH3), 66.05 (C1‴), 67.19 (C1), 70.30 (C2‴), 166.26 (C2″), 171.72 (C4″, 6″). Positive-ion FAB-MS m/z: 343 (M+H+). HR-FAB-MS m/z: 343.1982 (Calcd for C15H27N4O5: 343.1981). Anal. Calcd for C15H26N4O5·0.3H2O: C, 51.80; H, 7.71; N, 16.11. Found: C, 51.64; H, 7.85; N, 16.06.
1,1′-(6-Isopropoxy-1,3,5-triazine-2,4-diyl)bis(4-piperidinemethanol) (6bpp) and 4-[4-(Hydroxymethyl)piperidin-1-yl]-6-isopropoxy-1,3,5-triazin-2(5H)-one (7wbp) (Entry 6)These compounds were prepared from i-PrOH (bH) by method A under the conditions shown in Table 1. Purification of the products by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=95 : 4.7 : 0.3→85 : 14.5 : 0.5) gave 6bpp (84%) and 7wbp (5%) as a colorless solid and pale yellow solids, respectively. An analytical sample of 6bpp was obtained by recrystallization from EtCN as colorless crystals.
6bpp: mp 108–109°C (from EtCN). IR (KBr) cm−1: 3387 (OH of alcohol), 1571, 1515 (C=N), 1253, 1112, 1036 (C–O). 1H-NMR (CDCl3) δ: 1.17 (4H, m, H3′β, 5′β), 1.33 (6H, d, J=6.1 Hz, CH3), 1.72 (4H, m, H3′α, 5′α), 1.76 (2H, m, H4′α), 2.75 (2H, br s, OH), 2.78 (4H, t, J=12.5 Hz, H2′β, 6′β), 3.46 (4H, d, J=5.8 Hz, H1), 4.75 (4H, d, J=12.5 Hz, H2′α, 6′α), 5.23 (1H, qu, J=6.1 Hz, OCH<). 13C-NMR (CDCl3) δ: 21.77 (CH3), 28.44 (C3′, 5′), 38.87 (C4′), 43.11 (C2′, 6′), 67.17 (C1), 68.86 (OCH<), 165.66 (C2″, 4″), 170.15 (C6″). Positive-ion FAB-MS m/z: 366 (M+H+). HR-FAB-MS m/z: 366.2509 (Calcd for C18H32N5O3: 366.2505). Anal. Calcd for C18H31N5O3·0.25H2O: C, 58.43; H, 8.58; N, 18.93. Found: C, 58.43; H, 8.81; N, 18.79.
7wbp: mp 203–205°C. 1H-NMR (CDCl3) δ: 1.2 (2H, m, H3′β, 5′β), 1.35 (6H, d, J=6.4 Hz, CH3), 1.72 (2H, br s, OH), 1.85 (3H, m, H4′α, 3′α, 5′α), 2.89 (2H, m, H2′β, 6′β), 3.51 (2H, d, J=5.8 Hz, H1), 4.7, 4.9 (2H, br s, H2′α, 6′α), 5.29 (1H, qu, J=6.4 Hz, OCH<), 11.5 (1H, br s, NH). 13C-NMR (CDCl3) δ: 21.63 (CH3), 28.53 (C3′, 5′), 38.81 (C4′), 44.16 (C2′, 6′), 67.29 (C1), 72.25 (OCH<), 159.79, 161.51, 164.35 (C2″, 4″, 6″). 13C-NMR (DMSO-d6) δ: 21.37 (CH3), 28.41 (C3′, 5′), 38.26 (C4′), 43.46 (C2′, 6′), 65.38 (C1), 71.34 (OCH<), 156.83, 161.88, 163.39 (C2″, 4″, 6″). Positive-ion FAB-MS m/z: 269 (M+H+). HR-FAB-MS m/z: 269.1614 (Calcd for C12H21 N4O3: 269.1608).
[1-(4,6-Diisopropoxy-1,3,5-triazin-2-yl)piperidin-4-yl]methanol (7bbp) (Entry 7)This compound was prepared from bH by method A under the conditions shown in Table 1. Separation of the products by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=95 : 4.7 : 0.3→93 : 6.6 : 0.4) gave 7bbp (26%) and 6bpp (73%) as a pale yellow oil and pale yellow solid, respectively. An analytical sample of 7bbp was obtained by recrystallization from methylcyclohexane as pale yellow crystals.
7bbp: mp 95–96°C (from methylcyclohexane). IR (KBr) cm−1: 3398 (OH of alcohol), 1580, 1540 (C=N), 1273, 1129 (C–O). 1H-NMR (CDCl3) δ: 1.20 (2H, m, H3′β, 5′β), 1.36 (12H, d, J=6.4 Hz, CH3), 1.79 (4H, m, H3′α, 5′α, 4′α, OH), 2.85 (2H, dt, J=12.8, 2.4 Hz, H2′β, 6′β), 3.52 (2H, d, J=6.1 Hz, H1), 4.80 (2H, dm, J=13.4 Hz, H2′α, 6′α), 5.29 (1H, qu, J=6.4 Hz, OCH<). 13C-NMR (CDCl3) δ: 21.90 (CH3), 28.58 (C3′, 5′), 39.00 (C4′), 43.54 (C2′, 6′), 67.41 (C1), 70.08 (OCH<), 166.63 (C2″), 171.46 (C4″, 6″). Positive-ion FAB-MS m/z: 311 (M+H+). HR-FAB-MS m/z: 311.2080 (Calcd for C15H27N4O3: 311.2083). Anal. Calcd for C15H26N4O3·0.25H2O: C, 58.04; H, 8.44; N, 18.05. Found: C, 57.84; H, 8.56; N, 18.02.
4,6-Dichloro-2-isopropoxy-1,3,5-triazine (2b)12) (Entry 8): (Step 1)To a mixture of NaHCO3 (2.52 g, 30 mmol) and dry bH (2.88 g, 48 mmol) in dry CHCl3 (4 mL) was added compound 1 (1.84 g, 10 mmol) at room temperature with stirring. After stirring for 1 h at room temperature, the reaction mixture was refluxed for 17 h. After addition of CHCl3 (10 mL), the resulting mixture was washed with 1% aqueous NaHCO3 solution (10 mL×3) and the organic layer was dried with MgSO4. After evaporation of the solvent, the residue was separated by centrifugal chromatography (n-hexane : EtOAc=95 : 5) to give 2b (583 mg, 28% yield) as a colorless oil.
2b: IR (NaCl) cm−1: 2987 (CH), 1542, 1500 (C=N), 1100, 1036 (C–O of ether), 861, 807 (C–Cl). 1H-NMR (CDCl3) δ: 1.44 (6H, d, J=6.1 Hz, CH3), 5.41 (1H, qu, J=6.1 Hz, O–CH<). 13C-NMR (CDCl3) δ: 21.42 (CH3), 74.90 (O–CH<), 170.51 (C2), 172.47 (C4, 6). The positive FAB mass spectra of this compound showed no significant molecular ion to determine its chemical formula, indicating the instability of the corresponding molecular ion in the mass range.
Procedure for the Synthesis of 2,4,6-Triisopropoxy-1,3,5-triazine (4bbb)13) and 2-Chloro-4,6-diisopropoxy-1,3,5-triazine (3bb) (Method B)11) (Entry 9): (Step 1)To a mixture of NaHCO3 (5.04 g, 60 mmol) and dry bH (7.20 g, 120 mmol) in dry THF (40 mL) was added compound 1 (3.69 g, 20 mmol) at room temperature under an N2 atmosphere with stirring. After stirring for 7 d at room temperature, the reaction mixture was filtrated over celite and the filtrate was evaporated. To the residual oil was added CH2Cl2 (50 mL) and the separated insoluble material was removed by filtration. The solvent was evaporated and the residual oil was separated by centrifugal chromatography (n-hexane : EtOAc=98 : 2→90 : 10) to give 3bb (79 mg, 2%), 4bbb (442 mg, 9%).
4bbb: Colorless crystals, mp 92–94°C. IR (KBr) cm−1: 3433 (OH of H2O), 1563 (C=N), 1148, 1095 (C–O of ether). 1H-NMR (CDCl3) δ: 1.38 (18H, d, J=6.4 Hz, CH3), 5.35 (3H, qu, J=6.4 Hz, O–CH<). 13C-NMR (CDCl3) δ: 21.81 (CH3), 71.32 (O–CH<), 172.67 (C=N). Positive-ion FAB-MS m/z: 256 (M+H+). HR-FAB-MS m/z: 256.1658 (Calcd for C12H22N3O3: 256.1661). Anal. Calcd for C12H21N3O3·0.2H2O: C, 55.67; H, 8.33; N, 16.23. Found: C, 55.73; H, 8.35; N, 16.37.
3bb: 1H-NMR (CDCl3) δ: 1.40 (12H, d, J=6.4 Hz, CH3), 5.35 (2H, qu, J=6.4 Hz, O–CH<). 13C-NMR (CDCl3) δ: 21.63 (CH3), 71.97 (O–CH<), 171.63 (C2, 4), 172.61 (C6). Positive-ion FAB-MS m/z: 232 (M+H+). HR-FAB-MS m/z: 232.0852 (Calcd for C9H15ClN3O2: 232.0853).
[[6-(Pentan-3-yloxy)-1,3,5-triazine-2,4-diyl]bis(piperidine-1,4-diyl)]dimethanol (6cpp) (Entry 10)This compound was prepared from 3-pentanol (cH) by method A under the conditions shown in Table 1. Purification of the product by flash chromatography (n-hexane : i-PrOH=90 : 10→65 : 35) gave 6cpp (1.42g, 72%) as a white solid. An analytical sample of 6cpp was obtained by recrystallization from EtCN as colorless crystals.
6cpp: mp 114–116°C (from EtCN). IR (KBr) cm−1: 3399 (OH of alcohol), 1562, 1504 (C=N), 1249, 1112, 1035 (C–O). 1H-NMR (CDCl3) δ: 0.93 (6H, t, J=7.3 Hz, CH3), 1.18 (4H, m, H3′β, 5′β), 1.59 (2H, br s, OH), 1.67 (4H, m, CH2CH3), 1.77 (6H, m, H3′α, 5′α, 4′α), 2.79 (4H, dt, J=13.1, 2.1 Hz, H2′β, 6′β), 3.50 (4H, d, J=5.8 Hz, H1), 4.77 (4H, d, J=13.1 Hz, H2′α, 6′α), 4.98 (1H, m, OCH<). 13C-NMR (CDCl3) δ: 9.94 (CH3), 26.57 (CH2CH3), 28.57 (C3′, 5′), 39.16 (C4′), 43.27 (C2′, 6′), 67.67 (C1), 78.40 (OCH<), 166.01 (C2″, 4″), 171.16 (C6″). Positive-ion FAB-MS m/z: 394 (M+H+). HR-FAB-MS m/z: 394.2820 (Calcd for C20H36N5O3: 394.2818). Anal. Calcd for C20H35N5O3·1.2H2O: C, 57.86; H,9.08; N, 16.87. Found: C, 57.84; H, 8.87; N, 16.81.
General Procedure for the Preparation of Alkoxy-amino-1,3,5-triazine Derivatives (Method C) Example: [1-[4,6-Bis(pentan-3-yloxy)-1,3,5-triazin-2-yl]piperidin-4-yl]methanol (7ccp) (Entry 12): (Step 1)To a solution of cH (882 mg, 10.0 mmol) in dry THF (30 mL) was added n-BuLi (1.6 mol/L n-hexane solution, 6.25 mL, 10.0 mmol) at room temperature under an an N2 atmosphere. After stirring for 20 min at room temperature, a solution of compound 1 (922 mg, 5.0 mmol) in dry THF (10 mL) was added at room temperature. After additional stirring for 30 min at room temperature, the reaction mixture was refluxed for 2.5 h. After the solvent had been removed by evaporation, n-hexane (60 mL) and water (20 mL) were added. The organic layer was separated, dried (MgSO4), and evaporated. (Step 2) The residual yellow oil was dissolved in dry CH3CN (20 mL), and pH (922 mg, 8.0 mmol) was added, and the mixture was stirred for 30 min at room temperature. After filtration of the salt (pH·HCl) and evaporation of the solvent, the residue was separated by flash chromatography (n-hexane : i-PrOH : Et2NH=130 : 20 : 0.1) to give 7ccp (1.33 g, 73%) as a colorless oil.
7ccp: 1H-NMR (CDCl3) δ: 0.94 (12H, t, J=7.5 Hz, H1‴, 5‴), 1.22 (2H, m, H3′β, 5′β), 1.54 (1H, br s, OH), 1.70 (8H, m, H2‴, 4‴), 1.80 (3H, m, 4′α, 3′α, 5′α), 2.85 (2H, tm, J=13.1 Hz, H2′β, 6′β), 3.52 (2H, d, J=5.8 Hz, H1), 4.80 (2H, dm, J=13.1 Hz, H2′α, 6′α), 5.04 (2H, qu, J=6.7 Hz, H3‴). 13C-NMR (CDCl3) δ: 9.71 (C1‴, 5‴), 26.42 (C2‴, 4‴), 28.53 (C3′, 5′), 39.01 (C4′), 43.54 (C2′, 6′), 67.50 (C1), 79.29 (C3‴), 166.72 (C2″), 172.17 (C4″, 6″). Positive-ion FAB-MS m/z: 367 (M+H+). HR-FAB-MS m/z: 367.2710 (Calcd for C19H35N4O3: 367.2709).
[[6-(Cyclopentyloxy)-1,3,5-triazine-2,4-diyl]bis(piperidine-1,4-diyl)]dimethanol (6dpp) (Entry 14)This compound was prepared from cyclopentanol (dH) by method A under the conditions shown in Table 1. Purification of the product by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=97 : 2.7 : 0.3→90 : 9.5 : 0.5) gave 7ddp (25%) and 6dpp (67%) as a pale yellow oil and pale yellow solid, respectively. An analytical sample of 6dpp was obtained by recrystallization from CH3CN as pale yellow crystals.
6dpp: mp 178–179°C (from CH3CN). IR (KBr) cm−1: 3427 (OH of alcohol), 1568, 1519 (C=N), 1253, 1105, 1039 (C–O). 1H-NMR (CDCl3) δ: 1.18 (4H, m, H3′β, 5′β), 1.56 (2H, m, H3‴, 4‴), 1.59 (2H, br s, OH), 1.71–1.87 (10H, m, H4′α, 3‴, 4‴, 3′α, 5′α, 2‴, 5‴), 1.94 (2H, m, H2‴, 5‴), 2.79 (4H, dt, J=12.8, 12.1 Hz, H2′β, 6′β), 3.50 (4H, d, J=5.8 Hz, H1), 4.78 (4H, br d, J=12.8 Hz, H2′α, 6′α), 5.33 (1H, m, H1‴). 13C-NMR (CDCl3) δ: 24.04 (C3‴, 4‴), 28.58 (C3′, 5′), 32.77 (C2‴, 5‴), 39.14 (C4′), 43.26 (C2′, 6′), 67.65 (C1), 78.65 (C1‴), 165.90 (C2″, 4″), 170.71 (C6″). Positive-ion FAB-MS m/z: 392 (M+H+). HR-FAB-MS m/z: 392.2662 (Calcd for C20H34N5O3: 392.2662). Anal. Calcd for C20H33N5O3: C, 61.36; H, 8.50; N, 17.89. Found: C, 61.34; H, 8.70; N, 17.89.
[1-[4,6-Bis(cyclopentyloxy)-1,3,5-triazin-2-yl]piperidin-4-yl]methanol (7ddp) (Entry 15)This compound was prepared from dH by method C under the conditions shown in Table 1. Purification of the product by flash chromatography (n-hexane : i-PrOH : Et2NH=120 : 30 : 0.1) gave 7ddp (67%) as a colorless oil. An analytical sample of 7ddp was obtained by recrystallization from methylcyclohexane as colorless crystals. Formation of compound 5pp was also detected by TLC.
7ddp: mp 62–66°C (from methylcyclohexane). IR (KBr) cm−1: 3333 (OH of alcohol), 1584, 1544 (C=N), 1273, 1130, 1108, 1046 (C–O). 1H-NMR (CDCl3) δ: 1.21 (2H, m, H3′β, 5′β), 1.58 (5H, m, OH, H3‴, 4‴), 1.79 (11H, m, H4′α, 3‴, 4‴, 3′α, 5′α, 2‴, 5‴), 1.95 (4H, m, H2‴, 5‴), 2.85 (2H, dt, J=13.2, 2.7 Hz, H2′β, 6′β), 3.51 (2H, d, J=6.1 Hz, H1), 4.80 (2H, d, J=15.6 Hz, H2′α, 6′α), 5.40 (2H, m, H1‴). 13C-NMR (CDCl3) δ: 23.98 (C3‴, 4‴), 28.56 (C3′, 5′), 32.75 (C2‴, 5‴), 39.00 (C4′), 43.55 (C2′, 6′), 67.49 (C1), 79.56 (C1‴), 166.52 (C2″), 171.64 (C4″, 6″). Positive-ion FAB-MS m/z: 363 (M+H+). HR-FAB-MS m/z: 363.2397 (Calcd for C19H31N4O3: 363.2396). Anal. Calcd for C19H30N4O3·0.4C7H14: C, 65.17; H, 8.93; N, 13.95. Found: C, 65.07; H, 9.10; N, 13.68.
1,1′-(6-Cyclohexyloxy-1,3,5-triazine-2,4-diyl)bis[(piperidin-4-yl)methanol] (6epp) (Entry 16)This compound was prepared from cyclohexanol (eH) by method A under the conditions shown in Table 1. Purification of the product by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=930 : 66 : 4) gave 6epp (80%) as a yellow solid. Recrystallization from CH3CN gave an analytically pure product 6epp.
6epp: mp 172–173°C (from CH3CN). IR (KBr) cm−1: 3466, 3275 (OH of alcohol) 1252, 1038 (C–O of ether). 1H-NMR (CDCl3) δ: 1.18 (4H, m, H3′β, 5′β), 1.25 (1H, m, H4‴), 1.35 (2H, m, H3‴, 5‴), 1.51 (2H, m, H2‴, 6‴), 1.58 (1H, m, H4‴), 1.7–1.9 (10H, m, H4′, 3′α, 5′α, 3‴, 5‴, OH), 2.03 (2H, m, H2‴, 6‴), 2.79 (4H, dt, J=12.8, 2.1 Hz, H2′β, 6′β), 3.50 (4H, d, J=5.8 Hz, H1), 4.76 (4H, d, J=13.4 Hz, H2′α, 6′ α), 4.91 (1H, m, H1‴). 13C-NMR (CDCl3) δ: 24.28 (C3‴, 5‴), 25.56 (C4‴), 28.58 (C3′, 5′), 31.87 (C2‴, 6‴), 39.11 (C4′), 43.24 (C2′, 6′), 67.60 (C1), 74.54 (C1‴), 165.95 (C2″, 4″), 170.38 (C6″). Positive-ion FAB-MS m/z: 406 (M+H+). HR-FAB-MS m/z: 406.2820 (Calcd for C21H36N5O3: 406.2818). Anal. Calcd for C21H35N5O3: C, 62.20; H, 8.70; N, 17.27. Found: C, 62.04; H, 8.86; N, 17.16.
[1-[4,6-Bis(cyclohexyloxy)-1,3,5-triazin-2-yl]piperidin-4-yl]methanol (7eep) and [1-(6-Cyclohexyloxy-4-hydroxy-1,3,5-triazin-2-yl)piperidin-4-yl]methanol (7wep) (Entry 17)These compounds were prepared from eH by method A under the conditions shown in Table 1. Purification of the product by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=950 : 47 : 30→850 : 145 : 5) gave 7eep (54%) as a yellow solid, 6epp (22%) as a yellow solid, and 7wep (2%) as a white solid. Recrystallization from EtCN gave an analytically pure product 7eep as pale yellow needles.
7eep: mp 155–156°C (from EtCN). IR (KBr) cm−1: 3468 (OH of alcohol) 1149, 1107, 1040 (C–O of alcohol and ether). 1H-NMR (CDCl3) δ: 1.20 (2H, m, H3′β, 5′β), 1.25 (2H, m, H4‴), 1.37 (4H, m, H3‴, 5‴), 1.54 (4H, m, H2‴, 6‴), 1.58 (2H, m, H4‴), 1.6 (1H, br s, OH), 1.80 (7H, m, H3′α, 4′, 5′α, 3‴, 5‴, OH), 2.05 (4H, m, H2‴, 6‴), 2.85 (2H, dt, J=13.1, 2.4 Hz, H2′β, 6′β), 3.52 (2H, d, J=5.8 Hz, H1), 4.79 (2H, dm, J=13.1 Hz, H2′α, 6′α), 4.97 (2H, m, H1‴). 13C-NMR (CDCl3) δ: 24.10 (C3‴, 5‴), 25.47 (C4‴), 28.58 (C3′, 5′), 31.79 (C2‴, 6‴), 38.99 (C4′), 45.53 (C2′, 6′), 67.50 (C1), 75.37 (C1‴), 166.69 (C2″), 171.48 (C4″, 6″). Positive-ion FAB-MS m/z: 391 (M+H+). HR-FAB-MS m/z: 391.2709 (Calcd for C21H35N4O3: 391.2709). Anal. Calcd for C21H34N4O3: C, 64.59; H, 8.78; N, 14.35. Found: C, 64.58; H, 8.80; N, 14.38.
7wep: 1H-NMR (CDCl3) δ: 1.15–1.3 (3H, m, 3′β, 5′β, 4‴), 1.3–1.4 (2H, m, H3‴, 5‴), 1.5–1.6 (3H, m, H2‴, 4‴, 6‴), 1.7–1.9 (5H, m, H3′α, 4′, 5′α, 3‴, 5‴), 1.96 (2H, m, H2‴, 6‴), 2.23 (2H, br s, OH), 2.88 (2H, br t, J=11.9 Hz, H2′β, 6′β), 3.51 (2H, d, J=5.8 Hz, H1), 4.65–4.95 (2H, br m, H2′α, 6′α), 5.19 (1H, m, H1‴). 13C-NMR (CDCl3) δ: 23.63 (C3‴, 5‴), 25.20 (C4‴), 28.55 (C3′, 5′), 31.28 (C2‴, 6‴), 38.72 (C4′), 44.15 (C2′, 6′), 67.09 (C1), 75.29 (C1‴), 159.98 (C2″), 161.75 (C4″ or 6″), 163.90 (C6″ or 4″). Positive-ion FAB-MS m/z: 309 (M+H+). HR-FAB-MS m/z: 309.1929 (Calcd for C15H25N4O3: 309.1927).
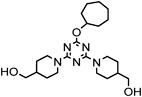
1,1′-(6-Cycloheptyloxy-1,3,5-triazin-2,4-diyl)bis-4-piperidinemethanol (6fpp) and [1-(6-Cycloheptyloxy)-4-hydroxy-1,3,5-triazin-2-yl)piperidin-4-yl]methanol (7wfp) (Entry 18)These compounds were prepared from cycloheptanol (fH) by method A under the conditions shown in Table 1. Purification of the products by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=950 : 47 : 3→930 : 66 : 4) gave 7ffp (38%) as a white solid, 6fpp (54%) as a white solid, and 7wfp (5%) as a reddish yellow solid. Recrystallization from EtCN gave an analytically pure product 6fpp as pale yellow crystals.
6fpp: mp 161–162°C (from EtCN). IR (KBr) cm−1: 3455 (OH of alcohol) 1252, 1094, 1037 (C–O of alcohol and ether). 1H-NMR (CDCl3) δ: 1.18 (4H, m, H3′β, 5′β), 1.45 (2H, m, H3‴, 6‴), 1.57 (4H, m, H4‴, 5‴), 1.66 (2H, br s, OH) 1.69–1.81 (10H, m, H3‴, 6‴, 4′, 3′α, 5′α, 2‴, 7‴), 2.05 (2H, m, H2‴, 7‴), 2.79 (4H, dt, J=13.1, 1.8 Hz, H2′β, 6′β), 3.51 (4H, d, J=5.8 Hz, H1), 4.77 (4H, dm, J=13.1 Hz, H2′α, 6′α), 5.09 (1H, m, H1‴). 13C-NMR (CDCl3) δ: 23.37 (C3‴, 6‴), 28.41 (C4‴, 5‴), 28.59 (C3′, 5′), 33.88 (C2‴, 7‴), 39.16 (C4′), 43.28 (C2′, 6′), 67.71 (C1), 77.05 (C1‴), 166.01 (C2″, 4″), 170.38 (C6″). Positive-ion FAB-MS m/z: 420 (M+H+). HR-FAB-MS m/z: 420.2974 (Calcd for C22H38N5O3: 420.2975). Anal. Calcd for C22H37N5O3: C, 62.98; H, 8.89; N, 16.69. Found: C, 62.91; H, 8.90; N, 16.74.
7wfp: Orange crystals, mp 186–188°C (from CH3CN). IR (KBr) cm−1: 3235 (OH of alcohol) 1297, 1103, 1045 (C–O of alcohol and ether). 1H-NMR (CDCl3) δ: 1.23 (2H, m, 3′β, 5′β), 1.45 (2H, m, H3‴, 6‴), 1.57 (4H, m, H4‴, 5‴), 1.71 (2H, m, H3‴, 6‴), 1.7–1.9 (6H, m, OH, H2‴, 7‴, 4′, 3′α, 5′α), 2.01 (2H, m, H2‴, 7‴), 2.89 (2H, m, H2′β, 6′β), 3.52 (2H, m, H1), 4.72 and 4.92 (2H, br s, H2′α, 6′α), 5.18 (1H, m, H1‴). 13C-NMR (CDCl3) δ: 22.86 (C3‴, 6‴), 28.30 (C4‴, 5‴), 28.44 (C3′, 5′), 33.46 (C2‴, 7‴), 38.81 (C4′), 43.17 (C2′, 6′), 67.29 (C1), 79.83 (C1‴), 159.62 (C2″), 161.30 (C4″ or C6″), 164.43 (C6″ or C4″). Positive-ion FAB-MS m/z: 323 (M+H+). HR-FAB-MS m/z: 323.2087 (Calcd for C16H27N4O3: 323.2083). Anal. Calcd for C16H26N4O3: C, 59.61; H, 8.13; N, 17.38. Found: C, 59.44; H, 8.25; N, 17.47.
[1-[4,6-Bis(cycloheptyloxy)-1,3,5-triazin-2-yl]piperidin-4-yl]-methanol (7ffp) (Entry 19)This compound was prepared from fH by method A under the conditions shown in Table 1. Purification of the product by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=950 : 47 : 3→930 : 66 : 4) gave 7ffp (52%) as a white solid and 6fpp (31%). Recrystallization from CH3CN gave an analytically pure product 7ffp as colorless needles.
7ffp: mp 127–128°C (from CH3CN). IR (KBr) cm−1: 3363 (OH of ROH) 1252, 1185, 1123 (C–O of ROH and ether). 1H-NMR (CDCl3) δ: 1.20 (2H, m, H3′β, 5′β), 1.46 (4H, m, H3‴, 6‴), 1.58 (8H, m, H4‴, 5‴), 1.68–1.84 (12H, m, H3‴, 6‴, 2‴, 7‴, 4′, 3′α, 5′α, OH), 2.04 (4H, m, H2‴, 7‴), 2.84 (2H, dt, J=13.1, 1.8 Hz, H2′β, 6′β), 3.51 (2H, d, J=5.8 Hz, H1), 4.79 (2H, dm, J=13.1 Hz, H2′α, 6′α), 5.15 (2H, m, H1‴). 13C-NMR (CDCl3) δ: 23.09 (C3‴, 6‴), 28.38 (C4‴, 5‴), 28.57 (C3′, 5′), 33.82 (C2‴, 7‴), 39.01 (C4′), 43.54 (C2′, 6′), 67.52 (C1), 77.84 (C1‴), 166.71 (C2″), 171.42 (C4″, 6″). Positive-ion FAB-MS m/z: 419 (M+H+). HR-FAB-MS m/z: 419.3018 (Calcd for C22H39N5O3: 419.3022). Anal. Calcd for C23H38N4O3: C, 66.00; H, 9.15; N, 13.39. Found: C, 65.79; H, 9.31; N, 13.35.
[1-[4,6-Bis(1,3-benzodioxol-5-yloxy)-1,3,5-triazin-2-yl]piperidin-4-yl]-methanol (7ggp) (Entry 21)This compound was prepared from sesamol (gH) by method A under the conditions shown in Table 1. Purification of the product by centrifugal chromatography (CH2Cl2 : EtOH=97 : 3) gave 7ggp (52%) as a white solid and 6gpp (16%) as a white solid. Recrystallization from EtCN gave an analytically pure product 7ggp as colorless crystals.
7ggp: mp 201–203°C (from EtCN). IR (KBr) cm−1: 3377 (OH of alcohol), 1242, 1177, 1137, 1038 (C–O of alcohol and ether). 1H-NMR (CDCl3) δ: 1.68 (2H, m, H3′β, 5′β), 1.34 (1H, br s, OH), 1.75 (3H, m, H4′α, 3′α, 5′α), 2.82 (2H, dt, J=12.8, 2.1 Hz, H2′β, 6′β), 3.51 (2H, d, J=5.8 Hz, H1), 4.62 (2H, d, J=13.4 Hz, H2′α, 6′α), 5.97 (4H, s, H2‴), 6.59 (2H, dd, J=8.2, 2.1 Hz, H6‴), 6.66 (2H, d, J=2.1 Hz, H4‴), 6.74 (2H, d, J=8.2 Hz, H7‴). 13C-NMR (CDCl3) δ: 28.45 (C3′, 5′), 38.81 (C4′), 43.75 (C2′, 6′), 67.35 (C1), 101.58 (C2‴), 104.04 (C4‴), 107.68 (C7‴), 114.11 (C6‴), 144.98 (C7‴a), 146.61 (C5‴), 147.73 (C3‴a), 166.53 (C2″), 172.53 (C4″, 6″). Positive-ion FAB-MS m/z: 467 (M+H+). HR-FAB-MS m/z: 467.1512 (Calcd for C23H23N4O7: 467.1567). Anal. Calcd for C23H22N4O7: C, 59.22; H, 4.75; N, 12.01. Found: C, 59.14; H, 4.82; N, 12.02.
Antiviral Activity Assay and Cytotoxicity of Target CompoundsThe antiviral activities of synthesized compounds were measured by using a plaque reduction assay16) as described in our previous paper.19) Results for antiviral activity (EC50) and cytotoxicity (IC50) with Vero cells are summarized in Table 2.